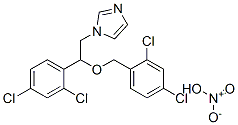
Eberconazole
- CAS NO.:128326-82-9
- Empirical Formula: C18H14Cl2N2
- Molecular Weight: 329.22
- MDL number: MFCD00865628
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-04 19:55:51
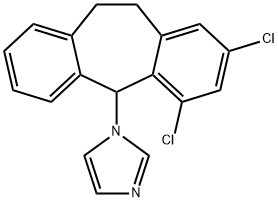
What is Eberconazole?
Description
Eberconazole is a new member of the azole class of antifungal agents, and it is indicated for the topical treatment of cutaneous fungal infections, including tinea corporis (ringworm of the body), tinea cruris (ringworm of the groin) and tinea pedis (athlete’s foot) infections. Its mode of action is similar to that of other azole antifungals, namely inhibition of fungal lanosterol 14α-demethylase. Eberconazole exhibits good in vitro activity against a wide range of Candida species, including Candida. tropicalis, dermatophytes and Malassezia spp. yeasts. It shows good activity against Candida. Parapsilosis (MIC90=0.125 mg/mL), which is a relevant species in skin and nail disorders. In addition, eberconazole is effective against some of the highly triazole-resistant yeasts such as Candida. glabrata and Candida. krusei, as well as fluconazole-resistant Candida. albicans. However, eberconazole is less active than clotrimazole and ketoconazole against Candida. neoformans and a number of clinically relevant molds. Eberconazole is supplied as a 1% or 2% cream, and the topical application does not result in detectable serum, urine, or fecal levels. In a phase II study of 60 patients with tinea corporis and tinea cruris, treatment with topical eberconazole (1% or 2% cream), applied once or twice daily for 6 weeks, resulted in cure rates ranging from 73.3–93.3% at the end of therapy, and 66.7–100% six weeks post-therapy.
Originator
Chiesi Wassermann (Spain)
The Uses of Eberconazole
Eberconazole can be used for a novel antifungal formulation.
Definition
ChEBI: 1-(2,4-dichloro-10,11-dihydrodibenzo[a,d][7]annulen-5-yl)imidazole is a member of the class of dibenzannulenes that is 10,11-dihydrodibenzo[a,d][7]annulene carrying two chloro substituents at positions 2 and 4 as well as an imidazol-1-yl substituent at position 5. It is a member of imidazoles, an organochlorine compound and a dibenzannulene. It derives from a hydride of a dibenzo[a,d][7]annulene.
brand name
Ebernet
Synthesis
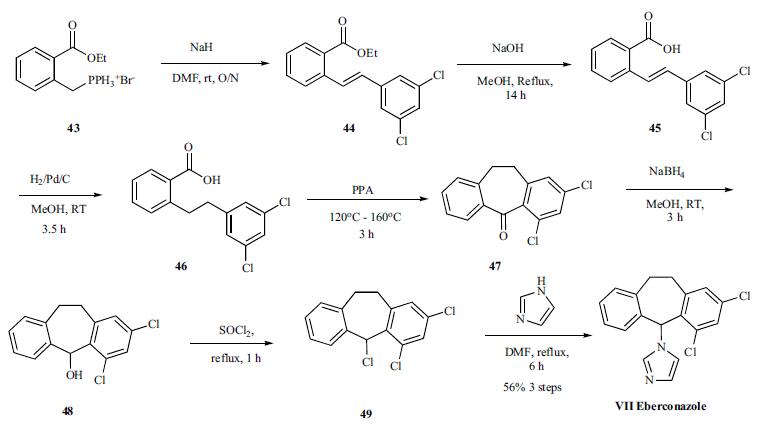
The synthesis of Eberconazole started with the Wittig reaction of the phosphonium bromide 43 with the 3,5-dichlorobenzaldehyde to give the olefin mixture 44. Hydrolysis of the ester followed by hydrogenation gives acid 46, which was cyclized to tricyclized ketone 47. Completion of the synthesis was accomplished in three steps via reduction of the ketone 47 with sodium borohydride, chlorination of resulting alcohol 48 with thionyl chloride and alkylation of the chloride 49 with imidazole to give eberconazole (VII).
Properties of Eberconazole
| Boiling point: | 495.0±45.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| pka | 5.84±0.10(Predicted) |
Safety information for Eberconazole
Computed Descriptors for Eberconazole
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 128326-82-9 Eberconazole 98%View Details
128326-82-9 Eberconazole 98%View Details
128326-82-9 -
 128326-82-9 98%View Details
128326-82-9 98%View Details
128326-82-9 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
