Dihydrostreptomycin
- CAS NO.:128-46-1
- Empirical Formula: C21H41N7O12
- Molecular Weight: 583.59
- MDL number: MFCD00084778
- EINECS: 204-888-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-10 15:54:08
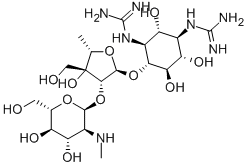
What is Dihydrostreptomycin?
Description
Dihydrostreptomycin was first synthesized by Parke Davis Co. in 1946by the reduction of streptomycin. Naturally occurring dihydrostreptomycin was found in the culture broth of Streptomyces humidus by Takeda Chemicals Industries in 1957. Its hydrochloride or sulfate is more easily crystallized and more stable under alkaline conditions than streptomycin. Dihydrostreptomycin has been used for therapy of tuberculosis, but because it has a higher ototoxicity than streptomycin its use is now restricted to animal therapy.
Originator
Dihydrostrepto,MSD ,US,1948
The Uses of Dihydrostreptomycin
Antibacterial.
Background
Dihydrostreptomycin is an aminoglycoside antibiotic. In humans, the use of dihydrostreptomycin has been associated with ototoxicity. The FDA withdrew its approval for the use of all drug products containing dihydrostreptomycin sulfate.
Definition
ChEBI: Dihydrostreptomycin is a member of streptomycins.
Manufacturing Process
Dihydrostreptomycin sulfate may be prepared from streptomycin sulfate by catalytic hydrogenation (Merck, Pfizer, Cyanamid), electrolytic reduction (Schenley, Olin Mathieson), or by sodium borohydride reduction (Bristol), or by isolation from a fermentation process (Takeda).
brand name
Abocillin;Biostrep;Complexobiotico;Diapenin 3;Diapenin balsamico;Diarrestival;Didromycin;Didrothenate;Dihydrocidan sulfato;Dihydrostreptofar;Diidro-pantostrept;Distreptopab;Dreiciclina balsamica;Dst;Entera-strept;Estreptoluy;Estreptosirup;Helle-strep-forte;Hp 48;Mastigun;Mixtencillin;Retromyopen;Rocopenstrep;Sanstrepto;Solmycin;Solvo-strept;Streptoduocin;Veticar;Veycil-as.
Therapeutic Function
Antibiotic
World Health Organization (WHO)
Dihydrostreptomycin, a derivative of the aminoglycoside antibiotic streptomycin with similar antibacterial activity, was first synthesized in 1947 and subsequently used in the treatment of tuberculosis and gram-negative infections. Preparations for systemic use have been widely withdrawn as a result of concern regarding their severe ototoxicity. Dihydrostreptomycin is poorly absorbed from the gastrointestinal tract. It remains available in oral preparations in some countries.
Safety Profile
Poison by intravenous and intramuscular routes. Moderately toxic by subcutaneous and intraperitoneal routes. Human teratogenic effects by unspecified route: developmental abnormahties of the eye and ear. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. A derivative of streptomycin; has anesthetic properties. When heated to decomposition it emits toxic fumes of NOx
Metabolism
Not Available
Properties of Dihydrostreptomycin
| Melting point: | >300 °C |
| Boiling point: | 641.09°C (rough estimate) |
| Density | 1.3963 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| pka | pKa 7.8 (Uncertain) |
Safety information for Dihydrostreptomycin
Computed Descriptors for Dihydrostreptomycin
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
