D-(-)-Salicin
Synonym(s):D -(−)-Salicin;2-(Hydroxymethyl)phenyl-β-D -glucopyranoside;Salicoside;Salicyl alcohol glucoside;Saligenin β-D -glucoside
- CAS NO.:138-52-3
- Empirical Formula: C13H18O7
- Molecular Weight: 286.28
- MDL number: MFCD00006590
- EINECS: 205-331-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 19:14:41
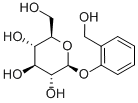
What is D-(-)-Salicin?
Chemical properties
white crystals or powder
The Uses of D-(-)-Salicin
D-(-)-Salicin has been used:
- to study its in vitro anticoagulant and antiplatelet activities
- as a standard in high performance liquid chromatography method (HPLC) for quantitation of salicin from willow plant
- as a tastant in taste threshold assay
- as a constituent of nutrient agar-salicin medium for selective isolation of Lactobacillus paracasei
The Uses of D-(-)-Salicin
analgesic, antipyretic
The Uses of D-(-)-Salicin
H1 antihistamine (nonsedating)
The Uses of D-(-)-Salicin
Standard substrate in evaluating enzyme Preparations contg b-glucosidase: Weidenhagen, Z. Ver. Zucker-Ind. 79, 591 (1929).
What are the applications of Application
D-(?)-Salicin is an inhibitor of Cox
Definition
ChEBI: An aryl beta-D-glucoside that is the beta-D-glucoside of the phenolic hydroxy group of salicyl alcohol.
General Description
Salicin is a non-phenolic glucosidic compound extracted from meadowsweet (Filipendula ulmaria L). It is majorly used as a substitute for quinine. Salicin can be used as a therapeutic for patients suffering from rheumatic fever. Salicin acts a metabolic precursor for salicylic acid. It is a novel phytochemical that exhibits immunological cross functions in plants and humans. Salicin facilitates growth and reproduction in plants. In addition, It also protects plants against biotic and abiotic stress.
Biochem/physiol Actions
D-(?)-Salicin exerts anti-rheumatism and anti-inflammatory activities. It inhibits lipopolysaccharide (LPS) induced inflammation in in vitro and in vivo studies. It regulates different signaling pathways such as nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK). It also regulates cytokines concentration such as tumor necrosis factor-alpha(TNF-α), interleukin-1β, interleukin-6, and interleukin-10.
Purification Methods
Crystallise D(-)-salicin from EtOAc, EtOH or water and sublime it at 190-195o/12mm. [Armour et al. J Chem Soc 412 1961, IR: Pearl & Darling J Org Chem 24 731 1959, Beilstein 17 III/IV 2986, 17/7 V 113.]
Properties of D-(-)-Salicin
| Melting point: | 196-202 °C |
| Boiling point: | 388.65°C (rough estimate) |
| alpha | -61.5 º (c=5, water) |
| Density | 1.4340 |
| refractive index | -62 ° (C=3, H2O) |
| storage temp. | 2-8°C |
| solubility | 36g/l |
| form | Fine Crystalline Powder |
| pka | 12.80±0.70(Predicted) |
| color | White |
| Water Solubility | 36 g/L (15 ºC), 250 g/L (60 ºC) |
| Merck | 14,8324 |
| BRN | 89593 |
| Stability: | Stable, but light sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 138-52-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Salicin(138-52-3) |
| EPA Substance Registry System | .beta.-D-Glucopyranoside, 2-(hydroxymethyl)phenyl (138-52-3) |
Safety information for D-(-)-Salicin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for D-(-)-Salicin
| InChIKey | NGFMICBWJRZIBI-MICYEWLZSA-N |
D-(-)-Salicin manufacturer
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 Salicin CAS 138-52-3View Details
Salicin CAS 138-52-3View Details
138-52-3 -
 Salicin 98% CAS 138-52-3View Details
Salicin 98% CAS 138-52-3View Details
138-52-3 -
 Salicin CAS 138-52-3View Details
Salicin CAS 138-52-3View Details
138-52-3 -
 SALICIN For Microbiology CAS 138-52-3View Details
SALICIN For Microbiology CAS 138-52-3View Details
138-52-3 -
 D-Salicin 99% CAS 138-52-3View Details
D-Salicin 99% CAS 138-52-3View Details
138-52-3 -
 D-(−)-Salicin CAS 138-52-3View Details
D-(−)-Salicin CAS 138-52-3View Details
138-52-3 -
 D-(−)-Salicin CAS 138-52-3View Details
D-(−)-Salicin CAS 138-52-3View Details
138-52-3 -
 D-Salicin 99%, Grade Standard: Technical Grade, Packaging Type: 1 KgView Details
D-Salicin 99%, Grade Standard: Technical Grade, Packaging Type: 1 KgView Details
138-52-3
