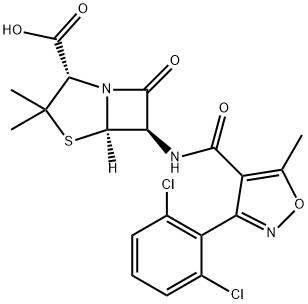
Cloxacillin
- CAS NO.:61-72-3
- Empirical Formula: C19H18ClN3O5S
- Molecular Weight: 435.88
- MDL number: MFCD00242583
- EINECS: 200-514-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-04 19:55:51
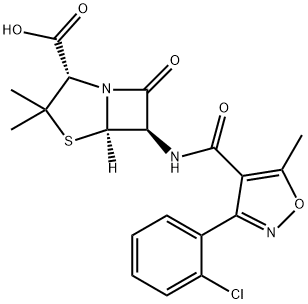
What is Cloxacillin?
Absorption
Well absorbed from the gastrointestinal tract.
Toxicity
Oral LD50 in rat and mouse is 5000 mg/kg. Intravenous LD50 in rat is 1660 mg/kg. Symptoms of overdose include wheezing, tightness in the chest, fever, itching, bad cough, blue skin color, fits, and swelling of face, lips, tongue, or throat.
Description
Cloxacillin induced contact dermatitis in a pharmaceutical factory worker with positive reactions to ampicillin but not to penicillin. The chemical formula of this is 3-o-chlorophenyl-5-methyl-4-isoxazolyl penicillin (Knudsen et al., 1962); this differs from oxacillin only by an additional chlorine atom.
Originator
Orbenin,Beecham,UK,1962
The Uses of Cloxacillin
Cloxacillin is a semisynthetic beta-lactamase resistant penicillin antibiotic with antibacterial activity. It is used to treat a wide variety of bacterial infections.
Indications
Cloxacillin is indicated for the treatment of beta-hemolytic streptococcal, pneumococcal, and staphylococcal infections (including beta-lactamase producing organisms).
Background
A semi-synthetic penicillin antibiotic which is a chlorinated derivative of oxacillin.
Definition
ChEBI: Cloxacillin is a semisynthetic penicillin antibiotic carrying a 3-(2-chlorophenyl)-5-methylisoxazole-4-carboxamido group at position 6. It has a role as an antibacterial agent and an antibacterial drug. It is a semisynthetic derivative, a penicillin allergen and a penicillin. It is functionally related to an oxacillin. It is a conjugate acid of a cloxacillin(1-).
Manufacturing Process
The reaction between 6-aminopenicillanic acid (6.5 g) and 3-o-chlorophenyl-5-
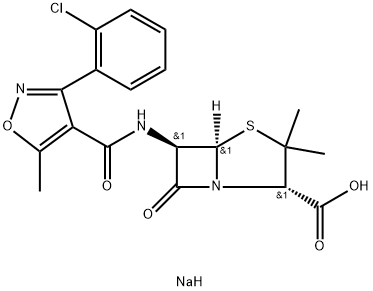
methylisoxazole4-carbonyl chloride (7.66 g) gave the sodium salt of 3-o-chlorophenyl-5-methyl-4-isoxazolylpenicillin(Cloxacillin) (9.98 g) as a pale yellow solid.
Colorimetric assay with hydroxylamine against a benzylpenicillin standard
indicated a purity of 68%.The 3-o-chlorophenyl-5-methylisoxazole-4-carboxylic acid, from which the acid
chloride was prepared, was obtained by hydrolysis of the ester product of the
reaction between o-chlorobenzohydroxamic chloride and ethyl acetoacetate in
methanolic sodium methoxide. Reaction with thionyl chloride gave the starting
material.
brand name
Allypropymal;Alurate sodium;Aprozal;Isonal;Nervisal;Numal;Somnipron.
Therapeutic Function
Antibacterial
World Health Organization (WHO)
Ampicillin and cloxacillin are listed separately in the WHO Model List of Essential Drugs.
Contact allergens
Cloxacillin is a semisynthetic penicillin close to oxacillin. It induced contact dermatitis in a pharmaceutical factory worker with positive reactions to ampicillin, but not to penicillin. In cutaneous drug reactions such as acute generalized exanthematous pustulosis due to amoxicillin, cross-reactivity is frequent to cloxacillin (personal observations).
Pharmacokinetics
Cloxacillin is a semisynthetic antibiotic in the same class as penicillin. Cloxacillin is for use against staphylococci that produce beta-lactamase.
Side Effects
Common side effects include Upset stomach, nausea, vomiting, diarrhea, gas, and mouth sores may occur.
Metabolism
Cloxacillin, like other penicillins, appears to be metabolized via breakage of the beta-lactam ring to form an inactive penicilloic acid metabolite.
Purification Methods
Purify mandelic acid by Soxhlet extraction with *C6H6 (about 6mL/g) and allow the extract to crystallise. It can also be recrystallised from CHCl3. The S-benzylisothiuronium salt has m 169o (166o) (from H2O). Dry it at room temperature under vacuum. [Beilstein 10 IV 565.]
Properties of Cloxacillin
| Boiling point: | 689.7±55.0 °C(Predicted) |
| Density | 1.2954 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| pka | pKa 2.70±0.07(H2O t=35.0 c=0.0025) (Uncertain) |
Safety information for Cloxacillin
Computed Descriptors for Cloxacillin
| InChIKey | LQOLIRLGBULYKD-JKIFEVAISA-N |
| SMILES | N12[C@@]([H])([C@H](NC(C3=C(C)ON=C3C3=CC=CC=C3Cl)=O)C1=O)SC(C)(C)[C@@H]2C(O)=O |
Cloxacillin manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 Cloxacillin 61-72-3 99%View Details
Cloxacillin 61-72-3 99%View Details
61-72-3 -
 61-72-3 Cloxacillin 98%View Details
61-72-3 Cloxacillin 98%View Details
61-72-3 -
 Cloxacillin IP/BP/USP Powder, PHARMAX LIFESCIENCES, 25 KGView Details
Cloxacillin IP/BP/USP Powder, PHARMAX LIFESCIENCES, 25 KGView Details
61-72-3 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
