Clortermine
- CAS NO.:10389-73-8
- Empirical Formula: C10H14ClN
- Molecular Weight: 183.68
- MDL number: MFCD00864481
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-22 08:28:48
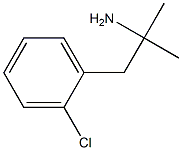
What is Clortermine?
Originator
Voranil,U.S.V.,US,1973
The Uses of Clortermine
Anorexic.
Definition
ChEBI: Clortermine is a member of amphetamines.
Manufacturing Process
To a Grignard reagent (prepared from 50.0 g of o,α-dichloro-toluene and 7.45
g of magnesium in diethyl ether) is added 18.0 g of acetone at such rate that
constant reflux is maintained. The reaction mixture is allowed to stand
overnight at room temperature, and is then poured onto a mixture of 20%
sulfuric acid and ice. The organic layer is separated, washed with water, an
aqueous solution of sodium hydrogen carbonate and again with water, dried
over magnesium sulfate and evaporated to dryness. The residue is distilled
under reduced pressure to yield 42.6 g of 1-(o-chlorophenyl)-2-methyl-2-
propanol, BP 120° to 122°C/12.5 mm.
To 29.0 ml of glacial acetic acid, cooled to 15°C, is added 11.5 g of sodium
cyanide (98%) while stirring, and then dropwise 32.4 ml of concentrated
sulfuric acid, dissolved in 29 ml of glacial acetic acid, while maintaining a
temperature of 20°C. The 1-(o-chlorophenyl)-2-methyl-2-propanol is added
moderately fast, allowing the temperature to rise spontaneously. After
completing the addition, the reaction mixture is heated to 70°C and stirred,
and is then poured onto a mixture of water and ice. The aqueous mixture is
neutralized with sodium carbonate and extracted with diethyl ether. The
organic solution is washed with water, dried over magnesium sulfate and
evaporated to dryness.
The oily residue is taken up in 100 ml of 6 N aqueous hydrochloric acid and
refluxed until a clear solution is obtained. The latter is made basic with
aqueous ammonia and extracted with diethyl ether; the organic solution is
separated, washed, dried and evaporated. The residue is distilled under
reduced pressure to yield 26.3 g of 1-(o-chlorophenyl)-2-methyl-2-
propylamine, BP 116° to 118°C/16 mm.
The 1-(o-chlorophenyl)-2-methyl-2-propylamine hydrochloride is prepared by
adding ethanolic hydrogen chloride to an ice-cold solution of the free base in
ethanol; the desired salt precipitates and is recrystallized from ethanol, MP
245° to 246°C.
Therapeutic Function
Antiobesity
Properties of Clortermine
| Boiling point: | bp16 116-118° |
| Density | 1.074±0.06 g/cm3(Predicted) |
| pka | 9.60±0.25(Predicted) |
Safety information for Clortermine
Computed Descriptors for Clortermine
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran





You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
