Citric acid monohydrate
Synonym(s):2-Hydroxypropane-1,2,3-tricarboxylic acid, Hydroxytricarballylic acid;Citric acid monohydrate
- CAS NO.:5949-29-1
- Empirical Formula: C6H10O8
- Molecular Weight: 210.1388
- MDL number: MFCD00008765
- EINECS: 200-662-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:19:11
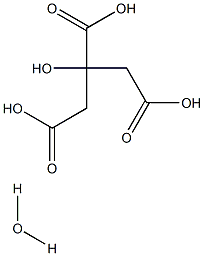
What is Citric acid monohydrate?
Chemical properties
Citric acid monohydrate appears as colorless or translucent crystals or a white crystalline powder. It is odorless with a strong acidic taste and has an orthorhombic crystal structure. It loses its water of crystallization in dry air or when heated to 40–50°C, softens at 75°C, and melts at around 100°C. It is used as a natural preservative and to add sour taste to foods and soft drinks. It also functions as a preservative, antioxidant, acidulant, flavoring agent, and anti-staling agent in products like fruit drinks, candy, cookies, biscuits, canned fruits, jams, and jellies. It contains 7.5–9.0% moisture, distinguishing it from other citric acid forms.
Description
Citric Acid Monohydrate is a white crystalline powder that functions as a preservative and antioxidant. It serves as an acidulant, flavoring agent, and anti-staling agent in various food products, including fruit drinks, candy, cookies, biscuits, canned fruits, jams, and jellies. It is distinguished from other forms of citric acid by its moisture content, which ranges from 7.5% to 9.0%.
The Uses of Citric acid monohydrate
Citric Acid Monohydrate, a tricarboxylic acid found in citrus fruits, has multiple uses. It serves as an acidulant and food additive, helping to control pH and enhance flavor. In pharmaceutical applications, it acts as an excipient with antioxidant properties, maintaining the stability of active ingredients and serving as a preservative. Additionally, it functions as an anticoagulant by chelating calcium in blood.
What are the applications of Application
Citric acid monohydrate has a wide range of applications. It is used to prepare citrate buffers for intravital microscopy and to unmask antigens and epitopes. It also serves as a pH-control agent in the food, beverage, and pharmaceutical industries. In animal nutrition, it enhances calcium utilization. Recent applications include its use as a carbon source in carbon nanodot synthesis, in preparing pH-6.0 solutions/buffers for tissue sample preparation, and in solubilization techniques for various samples.
Production Methods
Citric acid naturally occurs in various plant species and can be extracted from sources like lemon juice (containing 5–8% citric acid) or pineapple waste. Industrially, anhydrous citric acid is produced through mycological fermentation of crude sugar solutions, such as molasses, using strains of Aspergillus niger. Purification is achieved through recrystallization: the anhydrous form is obtained from a hot, concentrated aqueous solution, while the monohydrate form is derived from a cold, concentrated solution.
Properties of Citric acid monohydrate
| Melting point: | -94 °C(lit.) |
| Boiling point: | 56 °C760 mm Hg(lit.) |
| Density | 0.791 g/mL at 25 °C(lit.) |
| vapor density | 2 (vs air) |
| vapor pressure | 184 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 1 °F |
| storage temp. | no restrictions. |
| solubility | Citric Acid Monohydrate is very soluble in water, freely soluble in ethanol and sparingly soluble in ether. |
| form | Solid |
| pka | 3.138, 4.76, 6.401 |
| Specific Gravity | 0.810 (20/4℃) |
| color | White |
| PH | 1.85 (50g/l, H2O, 25℃) |
| Odor | Typical, practically odorless |
| Water Solubility | 1630 g/L (20 oC) ;H2O: soluble 54% (w/w) at 10°C (Citric acid in water) |
| Sensitive | Hygroscopic |
| Merck | 14,2326 |
| BRN | 4018641 |
| Stability: | Stable. Incompatible with oxidizing agents, bases, reducing agents, nitrates. |
| CAS DataBase Reference | 5949-29-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Citric acid monohydrate(5949-29-1) |
| EPA Substance Registry System | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, hydrate (1:1) (5949-29-1) |
Safety information for Citric acid monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Citric acid monohydrate
| InChIKey | YASYEJJMZJALEJ-UHFFFAOYSA-N |
Citric acid monohydrate manufacturer
JSK Chemicals
SGS & Company
H. K. Group
ASM Organics
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 CITRIC ACID MONOHYDRATE 99%View Details
CITRIC ACID MONOHYDRATE 99%View Details -
 Citric Acid Monohydrate 99%View Details
Citric Acid Monohydrate 99%View Details -
 CITRIC ACID MONOHYDRATE 99%View Details
CITRIC ACID MONOHYDRATE 99%View Details -
 Citric acid monohydrate 99%View Details
Citric acid monohydrate 99%View Details -
 Citric acid monohydrate 98%View Details
Citric acid monohydrate 98%View Details -
 Citric acid, monohydrate, 98% 99%View Details
Citric acid, monohydrate, 98% 99%View Details -
 Citric Acid Monohydrate CASView Details
Citric Acid Monohydrate CASView Details -
 Extran® AP 22 CASView Details
Extran® AP 22 CASView Details
