Cetuximab
- CAS NO.:205923-56-4
- Empirical Formula: C107H179N35O36S7
- Molecular Weight: 2756.23406
- MDL number: MFCD06407972
- Update Date: 2026-02-03 17:51:38
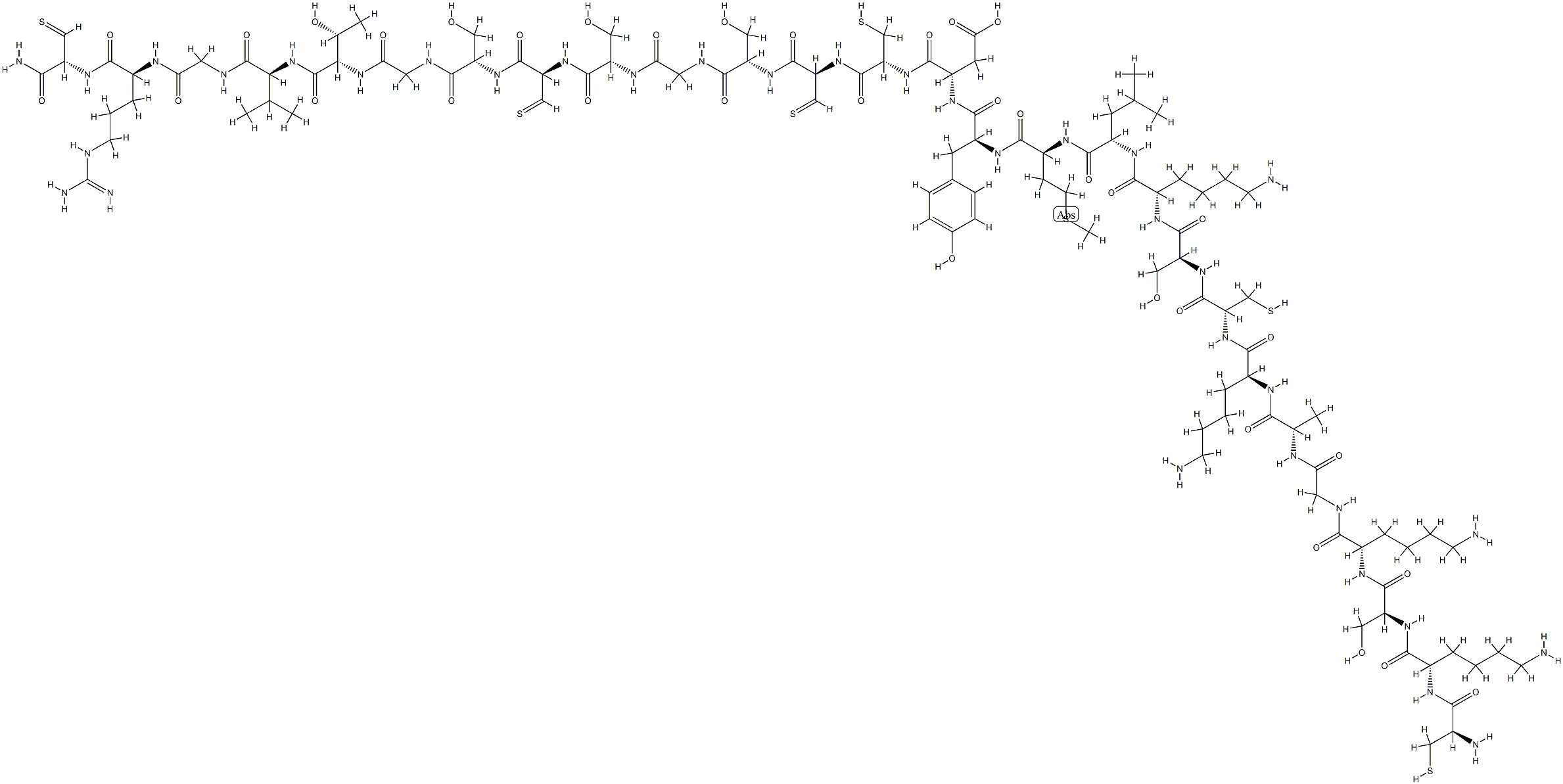
What is Cetuximab?
Description
Cetuximab, a human/mouse chimeric monoclonal antibody that blocks the EGFR, was launched for use in combination with irinotecan in the treatment of patients with colorectal cancer who no longer respond to standard chemotherapy treatment with irinotecan. Cetuximab is obtained by chimerization of M225, a murine anti-EGFR antibody; specifically heavy and light chains of the murine antibody are cloned and adapted for expression with constant regions of the human kappa light chain and human gamma1 heavy chain. It is produced by mammalian (suspension) cells in serum-free medium, purified by protein A affinity chromatography, ion-exchange chromatography, and gel filtration. Cetuximab binds specifically to EGFR on both normal and tumor cells. Overexpression of the human EGFR is detected in many cancers, including those of the colon and rectum. The binding of cetuximab to EGFR prevents growth factors from binding to the receptor, thereby inhibiting cell growth and inducing apoptosis. The therapeutic regimen of cetuximab consists of an initial loading dose of 400 mg/m2 administered as a 120-min IV infusion, followed by weekly maintenance dose of 250 mg/m2 infused over 60 min. The steady-state plasma concentrations of cetuximab are reached by the third weekly infusion and the mean elimination half-life is 114 h. Cetuximab is eliminated by binding to EGFRs in various tissues, followed by internalization of the antibody- EGFR complex. Systemic clearance of the antibody is saturated at higher doses, and this appears to correlate with saturation of EGFR binding. In a multicenter clinical trial in more than 300 patients with advanced metastatic colorectal cancer, combination therapy with cetuximab and irinotecan produced response in more than half of the patients, shrinking tumors in 23% and stopping tumor growth in an additional 33% of the patients. The most common adverse reactions associated with cetuximab were acneform rash, asthenia/malaise, fever, nausea, abdominal pain, constipation and vomiting. Serious adverse events such as infusion reaction, fever, sepsis, kidney failure, dehydration and diarrhea were experienced by <10% of the patients.
Originator
ImClone (US)
The Uses of Cetuximab
Treatment of EGF receptor-expressing cancers (monoclonal antibod.
brand name
Erbitux
Mechanism of action
Cetuximab, a monoclonal antibody, binds to the extracellular domain of the EGFR, which is overexpressed in many human cancers, including head, neck, and colorectal types. This process prevents the EGFR from binding with its endogenous ligand, blocking the receptor-dependent transduction pathway and providing many antitumor effects involving cell-cycle arrest, induction of apoptosis, inhibition of angiogenesis, inhibition of metastasis, internalization, and downregulation of the EGFR, and enhancement of the sensitivity to radiochemotherapy[1].
Clinical Use
Monoclonal antibody:
Treatment of EGFR-expressing metastatic colorectal
cancer in combination with irinotecan after failure of
irinotecan-including cytotoxic therapy
Treatment of head and neck cancer
Toxicology
The majority of skin toxicities were mild to moderate, symptomatic treatments were effective in controlling them. The skin discomfort caused by cetuximab can affect the quality of life temporarily; with long term treatment, severity of skin toxicity may decrease.
Drug interactions
Potentially hazardous interactions with other drugs
Avoid with live vaccines.
Metabolism
Several pathways have been described that may contribute to the metabolism of antibodies. All of these pathways involve the biodegradation of the antibody to smaller molecules, i.e. small peptides or amino acids.
References
[1] W Bou-Assaly, S Mukherji. “Cetuximab (erbitux).” American Journal of Neuroradiology 31 4 (2010): 626–7.
Properties of Cetuximab
| storage temp. | Store at 4°C, do not freeze |
| form | Liquid |
| color | Colorless to light yellow |
Safety information for Cetuximab
Computed Descriptors for Cetuximab
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Acebutolol EP Impurity K NLT 95%View Details
Acebutolol EP Impurity K NLT 95%View Details
74143-33-2 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 Ketamine Impurity-C NLT 95%View Details
90717-17-2 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
