cefsulodin sodium salt
- CAS NO.:52152-93-9
- Empirical Formula: C22H19N4O8S2.Na
- Molecular Weight: 554.53
- MDL number: MFCD00865072
- EINECS: 257-692-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39

What is cefsulodin sodium salt?
Description
Cefsulodin was synthesized by Takeda Chemicals Industries in 1974 by introducing the sulfobenzyl group, the same moiety as in sulbenicillin, at the 7 position of the cephem nucleus. Its side chain at the 3 position is similar to that of cephaloridine except for the carbamoyl group. The introduction of these hydrophilic groups increases the activity against Pseudomonas aeruginosa, but it markedly decreases it against gram-positive and other gramnegative bacteria. Therefore cefsulodin is used as a specific antibiotic against infections caused by the opportunistic pathogen P. aeruginosa.
Originator
Pseudomonil,Ciba Geigy,W. Germany,1980
The Uses of cefsulodin sodium salt
Cefsulodin is most commonly used in CIN agar to select for Yersinia microorganisms. The compound displays a mechanism of action like many β lactam antibiotics through inhibition of cell wall synthesis by competitively inhibiting penicillin binding protein (PBP) crosslinking of peptidoglycan resulting in inhibition of the final transpeptidation step. Through the inability for Cefsulodin sodium salt hydrate to inhibit cefsulodin-resistant mutants of Pseudomonas aeruginosa PAO4089 growth displayed that Cefsulodin sodium salt hydrate may compete with PBP3 in addition to PBP1A and PBP1B.
The Uses of cefsulodin sodium salt
betaadrenergic blocker
The Uses of cefsulodin sodium salt
Cefsulodin is a third generation cephalosporin (C258750) antibiotic designed specifically for Pseudomonas Aeruginosa.
The Uses of cefsulodin sodium salt
A β lactam antibiotic.
What are the applications of Application
Cefsulodin sodium salt is a β lactam antibiotic
Definition
ChEBI: Cefsulodin sodium is an organic molecular entity.
Manufacturing Process
0.514 g (4 x 10-3 mol) of 7-(α-sulfophenylacetamido)cephalosporanic acid, 0.466 g (3 x 10-3 mol) of isonicotinamide and 2.0 g (2.06 x 10-3 mol) of potassium thiocyanate were dissolved in 2.5 ml of water. The resulting solution was allowed to stand and heated for 20 hours in a thermostat kept at 50°C and then directly purified by chromatography on an Amberlite XAD-2 column (16 x 880 mm). Subsequently, the fractions containing the cephalosporins were collected and subjected to freeze-drying to obtain 270 g of the title product in the form of a pale yellowish white powder. The product is usually used as the sodium salt.
brand name
Cefomonil (TAP).
Therapeutic Function
Antibiotic
Biological Activity
cefsulodin, formerly named as sce-129, is a cephalosporin with a spectrum of antibacterial activity against staphylococcus aureus, pseudomonas aeruginosa, and most other gram-positive cocci [1]. cefsulodin shows little activity against other species of acinetobacter spp., pseudomonas, or members of the enterobacteriaceae [1]. cefsulodin is a β-lactam antibiotic that involved in lysing actively-growing e. coli by specifically binding to the intermembrane proteins, penicillin-binding proteins 1a and b, whose transglycosylase and transpeptidase activities are involved in cell elongation and septation [2].cefsulodin was active against p. aeruginos. cefsulodin was active against penicillinase-producing strains of s. aureus. the mics of cefsulodin for pseudomonas aeruginosa and its mutants pseudomonas aeruginosa pao4089 were 0·78 and 12· mg/l [3]. cefsulodin was active in minimum inhibitory concentrations (mics) of 0.5 to 64 μg/ml. cefsulodin was active against p. diminuta, p. maltophilia, p. paucimobilis, and p. pseudoalcaligenes with mics of 1-32 μg/ml. cefsulodin was not hydrolyzed by the β-lactamase induced in p. aeruginosa by growth in the presence of benzylpenicillin and was a poor substrate for β-lactamases from enterobacter cloacae and proteus morganii [4].
References
[1] barry a l, jones r n, thornsberry c. cefsulodin: antibacterial activity and tentative interpretive zone standards for the disk susceptibility test[j]. antimicrobial agents and chemotherapy, 1981, 20(4): 525-529.
[2] jacoby g h, young k d. cell cycle-independent lysis of escherichia coli by cefsulodin, an inhibitor of penicillin-binding proteins 1a and 1b[j]. journal of bacteriology, 1991, 173(1): 1-5.
[3] bryan l e, kwan s, godfrey a j. resistance of pseudomonas aeruginosa mutants with altered control of chromosomal beta-lactamase to piperacillin, ceftazidime, and cefsulodin[j]. antimicrobial agents and chemotherapy, 1984, 25(3): 382-384.
[4] king a, shannon k, phillips i. in vitro antibacterial activity and susceptibility of cefsulodin, an antipseudomonal cephalosporin, to beta-lactamases[j]. antimicrobial agents and chemotherapy, 1980, 17(2): 165-169.
Properties of cefsulodin sodium salt
| Melting point: | 175 C |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | H2O: 50 mg/mL, clear, light yellow |
| form | powder or crystals |
| color | Off-White to Pale Yellow |
| Water Solubility | Soluble in water. |
| Merck | 13,1958 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Pyridinium, 4-(aminocarbonyl)-1-[[(6R,7R)-2-carboxy-8-oxo-7-[[(2R)-phenylsulfoacetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-, inner salt, monosodium salt (52152-93-9) |
Safety information for cefsulodin sodium salt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for cefsulodin sodium salt
| InChIKey | REACMANCWHKJSM-DWBVFMGKSA-M |
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran
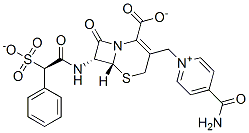

![sodium 2-[(2-aminoethyl)amino]ethanesulphonate](https://img.chemicalbook.in/CAS/GIF/34730-59-1.gif)
You may like
-
 Cefsulodin sodium salt hydrate 95% CAS 52152-93-9View Details
Cefsulodin sodium salt hydrate 95% CAS 52152-93-9View Details
52152-93-9 -
 Cefsulodin Sodium Salt (CFS) extrapure CAS 52152-93-9View Details
Cefsulodin Sodium Salt (CFS) extrapure CAS 52152-93-9View Details
52152-93-9 -
 Cefsulodin sodium salt hydrate CAS 52152-93-9View Details
Cefsulodin sodium salt hydrate CAS 52152-93-9View Details
52152-93-9 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
