CCPA
Synonym(s):CCPA
- CAS NO.:37739-05-2
- Empirical Formula: C15H20ClN5O4
- Molecular Weight: 369.8
- MDL number: MFCD00078574
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:36
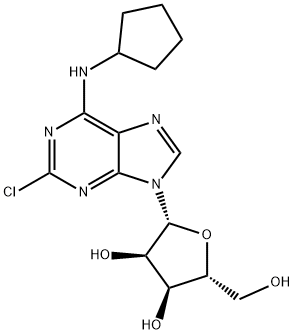
What is CCPA?
Chemical properties
Solid
The Uses of CCPA
Adenosine receptors are for treating central nervous system (CNS) disorders. Adenosine A1 receptor agonists include e.g. CCPA, 8-cyclopentyl-1,3-dipropylxanthine, R-phenylisopropyl adenosine. A2A rec eptor agonists include e.g. regadenoson (Lexiscan), apadenoson, binodenoson.
The Uses of CCPA
2-Chloro-N6-cyclopentyl Adenosine is a selective adenosine receptor agonist (1,2). It inhibits angiotensin II induced cardiomyocyte hypertrophy via the calcineurin signaling pathway (2).
The Uses of CCPA
2-Chloro-N6-cyclopentyladenosine (CCPA) may be used as an A1 adenosine receptor agonist with high selectivity. CCPA is often used with other adenosine receptor agonists and antagonist to resolve A(1), A(2A) and A(3) receptor functions. These include DPCPX (A(1) receptor antagonist), 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-α][1,3,5]triazin-5-ylamino]ethyl)phenol (A(2A) receptor antagonist), and 4-[2-[[6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride (A(2A) receptor agonist), and Cl-IB-MECA, (A(3)) receptor agonist).
What are the applications of Application
2-Chloro-N6-cyclopentyladenosine is a potent and selective Adenosine A1-R agonist
Biological Activity
Potent and selective adenosine A 1 receptor agonist (K i values are 0.8, 2300 and 42 nM for human A 1 , A 2A and A 3 receptors respectively; EC 50 = 18800 nM for hA 2B ). Centrally active following systemic administration in vivo .
Biochem/physiol Actions
A1 adenosine receptor agonist with high selectivity: reportedly 10,000-fold for A1 over A2 receptors.
Storage
Desiccate at RT
References
[1]. lohse mj, klotz kn, schwabe u, et al. 2-chloro-n6-cyclopentyladenosine: a highly selective agonist at a1 adenosine receptors. naunyn schmiedebergs arch pharmacol, 1988, 337(6): 687-689.
[2]. monopoli a, conti a, dionisotti s, et al. pharmacology of the highly selective a1 adenosine receptor agonist 2-chloro-n6-cyclopentyladenosine. arzneimittelforschung, 1994, 44(12): 1305-1312.
[3]. concas a, santoro g, mascia mp, et al. anticonvulsant doses of 2-chloro-n6-cyclopentyladenosine, an adenosine a1 receptor agonist, reduce gabaergic transmission in different areas of the mouse brain. j pharmacol exp ther, 1993, 267(2): 844-851.
[4]. sandoli d, chiu pj, chintala m, et al. in vivo and ex vivo effects of adenosine a1 and a2 receptor agonists on platelet aggregation in the rabbit. eur j pharmacol, 1994, 259(1): 43-49.
Properties of CCPA
| Boiling point: | 656.1±65.0 °C(Predicted) |
| Density | 1.87±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: 1.7 mg/mL, insoluble |
| form | solid |
| pka | 13.06±0.70(Predicted) |
| color | off-white to light yellow |
Safety information for CCPA
Computed Descriptors for CCPA
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
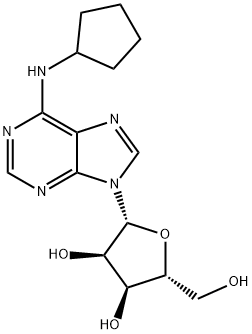

You may like
-
 2-Chloro-N6-cyclopentyladenosine CAS 37739-05-2View Details
2-Chloro-N6-cyclopentyladenosine CAS 37739-05-2View Details
37739-05-2 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
