Butenafine
- CAS NO.:101828-21-1
- Empirical Formula: C23H27N
- Molecular Weight: 317.47
- MDL number: MFCD00865577
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-17 18:22:24
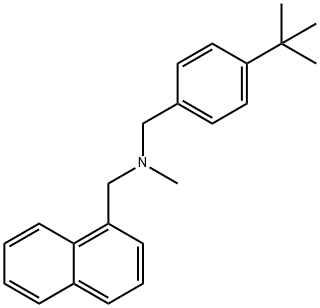
What is Butenafine?
Absorption
The total amount absorbed through the skin into the systemic circulation has not been quantified.
The Uses of Butenafine
Butenafine (Mentax) is a synthetic benzylamine antifungal agent, related structurally and pharmacologically to terbinafine. It is thought to act by inhibiting the enzyme squalene 2,3-epoxidase, resulting in decreased ergosterol and corresponding increase in squalene, with subsequent cell membrane permeability alteration and cell death. It is active against dermatophytes, yeast, and Sporothrix.

Background
Butenafine hydrochloride is a synthetic benzylamine antifungal agent. Butenafine's mechanism of action is believed to involve the synthesis inhibition of sterols. In particular, butenafine acts to inhibit the activity of the squalene epoxidase enzyme that is essential in the formation of sterols necessary for fungal cell membranes.
Indications
For the topical treatment of the following dermatologic infections: tinea (pityriasis) versicolor due to M. furfur, interdigital tinea pedis (athlete’s foot), tinea corporis (ringworm) and tinea cruris (jock itch) due to E. floccosum, T. mentagrophytes, T. rubrum, and T. tonsurans.
Definition
ChEBI: Trimethylamine in which hydrogen atoms attached to different methyl groups are substituted by 1-naphthyl and 4-tert-butylphenyl groups. It is an inhibitor of squalene epoxidase, an enzyme responsible for the creation of sterols needed in funga cell membranes, and is used as its hydrochloride salt for treatment of dermatological fungal infections.
brand name
Lotrimin (Schering); Mentax (Mylan Bertek).
Pharmacokinetics
Butenafine is a synthetic antifungal agent that is structurally and pharmacologically related to allylamine antifungals. The exact mechanism of action has not been established, but it is suggested that butenafine's antifungal activity is exerted through the alteration of cellular membranes, which results in increased membrane permeability, and growth inhibition. Butenafine is mainly active against dermatophytes and has superior fungicidal activity against this group of fungi when compared to that of terbinafine, naftifine, tolnaftate, clotrimazole, and bifonazole. It is also active against Candida albicans and this activity is superior to that of terbinafine and naftifine. Butenafine also generates low MICs for Cryptococcus neoformans and Aspergillus spp. as well.
Metabolism
The primary metabolite in urine was formed through hydroxylation at the terminal t-butyl side-chain.
Properties of Butenafine
| Melting point: | 200-202 °C |
| Boiling point: | 426.1±14.0 °C(Predicted) |
| Density | 1.032±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: 63 mg/mL (198.44 mM);Ethanol: 63 mg/mL (198.44 mM) |
| pka | 7.87±0.50(Predicted) |
| Water Solubility | Water: Insoluble |
| CAS DataBase Reference | 101828-21-1(CAS DataBase Reference) |
Safety information for Butenafine
Computed Descriptors for Butenafine
Butenafine manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
