Butafosfan
- CAS NO.:17316-67-5
- Empirical Formula: C7H18NO2P
- Molecular Weight: 179.2
- MDL number: MFCD00869071
- EINECS: 241-341-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-03 19:45:19
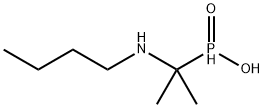
What is Butafosfan?
Chemical properties
Butafosfan is a white crystalline powder, very easily is dissolved in water. soluble in organic solvents such as ethanol, which should be purged with an inert gas. The solubility of butafosfan in ethanol is approximately 1 mg/ml. It has the effect that improves energy metabolism and strengthen immunologic function.
The Uses of Butafosfan
Butafosfan is an organic phosphorus supplement that is given, most commonly with cyanocobalamin, to cattle, swine, horses, and poultry for the prevention or treatment of deficiencies. When given with cyanocobalamin, butafosfan alters lipid metabolism, serving to decrease the prevalence of subclinical ketosis.
Benefits
Butafosfan works by increasing the body's ability to metabolise glucose and other nutrients, as well as supporting the production of adenosine triphosphate, which is the main source of cellular energy. Cyanocobalamin, a form of vitamin B12, is essential for normal nerve function and red blood cell formation.
Mechanism of action
Butafosfan works by improving the body’s ability to metabolize glucose and other nutrients, as well as by supporting the production of ATP, which is the primary source of energy for cells. The association of Butafosfan and cyanocobalamin could favors the phosphorylation of molecules that intermediate metabolic pathways, such as a gluconeogenesis, glycolysis, and the Krebs cycle, improving the synthesis of the co-nutrients (ATP and ADP mainly) and consequently increasing blood glucose. However, it is still unclear if these results refer mainly to one of the nutrients, Butafosfan or cyanocobalamin[1].
Side Effects
Common side effects may include indigestion, such as vomiting or diarrhoea, and local irritation at the injection site. In rare cases, more serious side effects, such as allergic reactions or neurological damage, may occur.
Toxicity evaluation
The acute toxicity of butafosfan is very low. The oral LD50 in mice was approximately 16 000 mg/kg bw. After parenteral administrations, the following LD50 values were obtained in mice: approximately 21 000 mg/kg bw (subcutaneously), approximately 10 000 mg/kg bw (intravenously) and greater than 2500 mg/kg bw (intraperitoneally). Mice dosed orally or subcutaneously died within 3 and 2 days, respectively, while survivors had recovered by that time. The LD50 value in chicken (intramuscularly) was 9974 mg/kg bw. Observed toxic signs were excitation, cyanosis, sternal recumbency and diarrhoea.
References
[1] Weiller M, et al. Butaphosphan Effects on Glucose Metabolism Involve Insulin Signaling and Depends on Nutritional Plan. Nutrients, 2020; 12: 1856.
Properties of Butafosfan
| Melting point: | 219 °C |
| Boiling point: | 273.4±42.0 °C(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | 2.99±0.10(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C7H18NO2P/c1-4-5-6-8-7(2,3)11(9)10/h8,11H,4-6H2,1-3H3,(H,9,10) |
| CAS DataBase Reference | 17316-67-5(CAS DataBase Reference) |
Safety information for Butafosfan
Computed Descriptors for Butafosfan
| InChIKey | WCXWARPXYQCRPP-UHFFFAOYSA-N |
| SMILES | P(C(NCCCC)(C)C)(O)=O |
Butafosfan manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran



You may like
-
 17316-67-5 ButaphosphanView Details
17316-67-5 ButaphosphanView Details
17316-67-5 -
 BUTAPHOSPHAN 99%View Details
BUTAPHOSPHAN 99%View Details -
 Butafosfan 98% (HPLC) CAS 17316-67-5View Details
Butafosfan 98% (HPLC) CAS 17316-67-5View Details
17316-67-5 -
 Butafosfan 98% CAS 17316-67-5View Details
Butafosfan 98% CAS 17316-67-5View Details
17316-67-5 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
