Bromonitromethane
- CAS NO.:563-70-2
- Empirical Formula: CH2BrNO2
- Molecular Weight: 139.94
- MDL number: MFCD00007401
- EINECS: 209-258-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:21
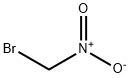
What is Bromonitromethane?
Chemical properties
Bromonitromethane is a clear yellow liquid, It is a strong oxidizing agent with properties similar to nitromethane.
Bromonitromethane has been used:
in production of various protected α-bromo nitroalkane donors (including Fmoc) for use in Umpolung amide synthesis.
in preparation of (Z)-1-bromo-1-nitroalkenes via sodium iodide-catalyzed Henry reaction.
in diastereo- and enantioselective cyclopropanation of β,γ-unsaturated a-ketoesters via domino Michael-addition/intramolecular-alkylation strategy.
The Uses of Bromonitromethane
Bromonitromethane has received considerable attention as a one-carbon synthon for the synthesis of a variety of important organic intermediates.
Bromonitromethane is a key starting material for the preparation of the broad-spectrum antibiotic trovafloxacin, which contains the interesting 3-azabicyclo[ 3.1.0]hexane ring system.
it was used in the synthesis of 2-nitrobenzofuran and 2-nitro-2,3-dihydrobenzofuran-3-ols, nitrobenzothio-phenes, and nitrothiazoles, polyfunctionalized nitrocyclopropanes. It has also been utilized in the synthesis of 1-bromo-1-nitroalkan-2-ols and aryl nitromethanes. In addition, it could be used as a bromine donor.
The Uses of Bromonitromethane
Bromonitromethane has been used:
- in production of various protected α-bromo nitroalkane donors (including Fmoc) for use in Umpolung amide synthesis
- in preparation of (Z)-1-bromo-1-nitroalkenes via sodium iodide-catalyzed Henry reaction
- in diastereo- and enantioselective cyclopropanation of β,γ-unsaturated a-ketoesters via domino Michael-addition/intramolecular-alkylation strategy
What are the applications of Application
Bromonitromethane is used in production of various protected α-bromo nitroalkane donors
Preparation
Bromonitromethane is commercially available and can also be easily prepared according to the procedures reported by Fishwick et al. A typical procedure is as following: freshly distilled nitromethane was stirred at 0℃ and bromine was dropped in 5 seconds. The resulted bromonitromethane could be used without further purification.
Properties of Bromonitromethane
| Melting point: | -28 °C |
| Boiling point: | 146-148 °C/750 mmHg (lit.) |
| Density | 2.007 g/mL at 25 °C (lit.) |
| refractive index | 1.485-1.495 |
| Flash point: | 4°C |
| solubility | Chloroform (Soluble), DMSO (Sparingly), Ethyl Acetate (Slightly), Methanol (Slig |
| form | Liquid |
| pka | 7.22±0.29(Predicted) |
| color | Clear yellow |
| Stability: | Light Sensitive, Volatile |
| CAS DataBase Reference | 563-70-2(CAS DataBase Reference) |
| EPA Substance Registry System | Methane, bromonitro- (563-70-2) |
Safety information for Bromonitromethane
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bromonitromethane
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Bromonitromethane CAS 563-70-2View Details
Bromonitromethane CAS 563-70-2View Details
563-70-2 -
 Bromonitromethane 92% CAS 563-70-2View Details
Bromonitromethane 92% CAS 563-70-2View Details
563-70-2 -
 Bromonitromethane, tech., 90% CAS 563-70-2View Details
Bromonitromethane, tech., 90% CAS 563-70-2View Details
563-70-2 -
 Bromonitromethane CAS 563-70-2View Details
Bromonitromethane CAS 563-70-2View Details
563-70-2 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
