Bromfenac Sodium
- CAS NO.:120638-55-3
- Empirical Formula: C15H11BrNNaO3
- Molecular Weight: 356.15
- MDL number: MFCD23843787
- EINECS: 695-342-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-28 14:58:11
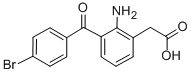
What is Bromfenac Sodium?
Description
Duract was launched in the US as a potent, orally-active, long lasting peripheral analgesic with antiinflammatory properties. It is structurally similar to ketoprofen and diclofenac and can be prepared in three steps from 2-amino-4’-bromophenone using Gassman’s oxindole synthesis. Duract’s biological effects are a result of its ability to reduce prostaglandin production through inhibition of cyclooxygenase. As a 4-bromo derivative of Amfenac, this modification increased the duration of analgesic activity and antiinflammatory potency. It was also free of any CNS, cardiovascular or autonomic effects. In comparison, 5 mg of Duract was equipotent to 650 mg of ASA and 25 mg was slightly more potent than 400 mg of Ibuprofen.
Chemical properties
Yellow Solid
Originator
American Home Products (US)
The Uses of Bromfenac Sodium
Bromfenac is a non-steroidal anti-inflammatory agent.
The Uses of Bromfenac Sodium
Bromfenac (Xibrom, ISTA Pharmaceuticals, Irvine, USA; Bronuck, Senju Pharmaceutical, Osaka, Japan) is indicated for the treatment of postoperative inflammation and the reduction of ocular pain in patients after undergoing cataract extraction. For this task, one drop of Xibrom may be applied to the affected eye twice daily beginning 24 hours after cataract surgery and continuing for the first 2 weeks of the postoperative period. The clinical safety and efficacy of bromfenac have been extensively studied in diverse comparative investigations, including the treatment of external or anterior ocular inflammatory diseases, allergic conjunctivitis, scleritis, and postoperative inflammation.The results of two phase III multicenter, randomized double-masked placebo-controlled clinical trials showed that bromfenac ophthalmic solution 0.09% was effective in the rapid resolution of ocular pain after cataract surgery, and there was a statistically significant difference between the bromfenac and placebo groups demonstrated in these phase III clinical trials.
Definition
ChEBI: The sesquihydrate of the sodium salt of bromfenac. It is used for the management of ocular pain and treatment of postoperative inflammation in patients who have undergone cataract extraction.
brand name
Xibrom (Ista).
Properties of Bromfenac Sodium
| Melting point: | 268-270°C (dec.) |
| storage temp. | Refrigerator |
| solubility | DMSO: ≥ 100 mg/mL (260.98 mM); Water: ≥ 100 mg/mL (260.98 mM) |
| form | Solid |
| color | Light yellow to yellow |
Safety information for Bromfenac Sodium
Computed Descriptors for Bromfenac Sodium
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran



You may like
-
 120638-55-3 Bromfenac Sodium 98%View Details
120638-55-3 Bromfenac Sodium 98%View Details
120638-55-3 -
 Bromfenac Sodium 98%View Details
Bromfenac Sodium 98%View Details -
 Bromfenac Sodium 99%View Details
Bromfenac Sodium 99%View Details
120638-55-3 -
 Bromfenac sodium sesquihydrate 98%View Details
Bromfenac sodium sesquihydrate 98%View Details
120638-55-3 -
 Bromfenac monosodium salt sesquihydrate 98% (HPLC) CAS 120638-55-3View Details
Bromfenac monosodium salt sesquihydrate 98% (HPLC) CAS 120638-55-3View Details
120638-55-3 -
 0.09% Bromfenac Ophthalmic Solution, 5 mlView Details
0.09% Bromfenac Ophthalmic Solution, 5 mlView Details
120638-55-3 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
