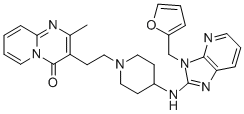
Barmastine
- CAS NO.:99156-66-8
- Empirical Formula: C27H29N7O2
- Molecular Weight: 483.573
- MDL number: MFCD00864643
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
What is Barmastine?
Originator
Barmastine,ZYF Pharm Chemical
The Uses of Barmastine
Antihistaminic.
Manufacturing Process
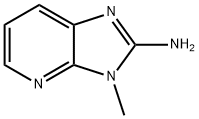
A mixture of N-(3-nitro-2-pyridinyl)-2-furanylmethanamine, of a solution of
thiophene in ethanone 4% and of methanol saturated with ammonia was
hydrogenated at normal pressure and at room temperature with platinum-on-charcoal catalyst 5%. After the calculated amount of hydrogen was taken up,
the catalyst was filtered off and the filtrate was evaporated. The residue was
crystallized from acetonitrile, yielding the N2-(2-furanylmethyl)-2,3-
pyridinediamine.
A mixture of 54 parts of ethyl 4-isothiocyanato-1-piperidinecarboxylate, 48
parts of N2-(2-furanylmethyl)-2,3-pyridinediamine and 450 parts of
tetrahydrofuran was stirred and refluxed overnight. The reaction mixture was
evaporated and the residue was crystallized from a mixture of 2-propanone
and 2,2'-oxybispropane. The product was filtered off and dried, yielding 76
parts (75%) of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3-
pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate; melting point
132.7°C.
A mixture of 74 parts of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3-
pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate, 96 parts of
mercury (II) oxide, 0.1 parts of sulfur and 800 parts of ethanol was stirred
and refluxed for 3 h. The reaction mixture was filtered over Hyflo and the
filtrate was evaporated to give 52.5 parts (79%) of ethyl 4-[[3-(2-
furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl]amino]-1-piperidinecarboxylate;
melting point 149.2°C (crystallized from acetonitrile).
A mixture of ethyl 4-[[3-(2-furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-
yl]amino]-1-piperidinecarboxylate and of a hydrobromic acid solution 48% in
water was stirred and heated for 3 h at about 80°C. The reaction mixture was
evaporated and the residue was dried, yielding the 3-(2-furanylmethyl)-N-(4-
piperidinyl)-3H-imidazo[4,5-b]pyridin-2-amine dihydrobromide (crystallized
from methanol).
A mixture of 3-(2-hydroxyethyl)-2-methyl-pyrido[2,1-b]pyrimidin-4-one, of
acetic acid and of a hydrobromic acid solution 67% in acetic acid was stirred
and heated to reflux. Stirring was continued overnight at reflux temperature.
The reaction mixture was evaporated and the solid residue was triturated in 2-
propanone. The product was filtered off and dried, yielding 3-(2-bromoethyl)-
2-methyl-pyrido[1,2-a]pyrimidin-4-one monohydrobromide.
A mixture of 3-(2-bromoethyl)-2-methyl-pyrido[1,2-a]pyrimidin-4-one
monohydrobromide, 3-furan-2-yl-methyl-(3H-imidazo[4,5-b]pyridine-2yl)-4-
piperidinyl)-amine dihydrobromide, of sodium carbonate and of N,Ndimethylformamide
was stirred and heated overnight at about 70°C. The
reaction mixture was poured onto water. The product was extracted with
trichloromethane. The extract was dried, filtered and evaporated. The residue
was purified by column chromatography over silica gel using a mixture of
trichloromethane and methanol (94:6 by volume), saturated with ammonia,
as eluent. The pure fractions were collected and the eluent was evaporated.
The residue was crystallized from acetonitrile, yielding 3-(2-(4-((3-(2-
furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl)amino)-1-piperidinyl)ethyl)-2-
methyl-4H-pyrido[1,2-a]pyrimidin-4-one; melting point 202°C.
Therapeutic Function
Antihistaminic
Safety information for Barmastine
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5

![1H-Imidazo[4,5-b]pyridin-2-amine(9CI)](https://img.chemicalbook.in/CAS/GIF/132898-03-4.gif)