Azaconazole
Synonym(s):1-[2-(2,4-Dichlorophenyl)-1,3-dioxolan-2-ylmethyl]-1H-1,2,4-triazole
- CAS NO.:60207-31-0
- Empirical Formula: C12H11Cl2N3O2
- Molecular Weight: 300.14
- MDL number: MFCD00865600
- EINECS: 262-102-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:18:34
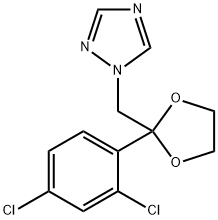
What is Azaconazole?
Originator
Azaconazole,Chemical
The Uses of Azaconazole
Azaconazole is particularly active against wood-destroying and sapstain fungi. It is also used as a disinfectant in mushroom cultivation and on storage boxes for fruit and vegetables.
The Uses of Azaconazole
Fungicide for cultivation on wood; preservative for composite wood products.
Definition
ChEBI: A member of the class of dioxolanes that is 1,3-dioxolane substituted at position 2 by 2,4-dichlorophenyl and 1,2,4-triazol-1-ylmethyl groups. A fungicide used mainly in ornamental crops to control canker and other diseases. Azaconazole is moderately toxic to mammals but is not expected to bioaccumulate. It is moderately toxic to birds, fish and aquatic invertebrates.
Manufacturing Process
A stirred and cooled (0°C) solution of 1-(2,4-diaminophenyl)-1-ethanone in a
concentrated hydrochloric acid solution, water and acetic acid was diazotated
with a solution of sodium nitrite in water. After stirring at 0°C, the whole was
poured onto a solution of copper (I) chloride in a concentrated hydrochloric
acid solution while stirring. The mixture was heated at 60°C. After cooling to
room temperature, the product was extracted twice with 2,2'-oxybispropane.
The combined extracts were washed successively with water, a diluted sodium
hydroxide solution and again twice with water, dried, filtered and evaporated,
yielding 1-(2,4-dichlorophenyl)-1-ethanone.
1-(2,4-Dichlorophenyl)-1-ethanon were dissolved in 1,2-ethanediol at heating.
While stirring bromine were added dropwise, without external heating. After
stirring at room temperature, 4-methylbenzenesulfonic acid and benzene were
added. The whole was stirred and refluxed overnight with water-separator.
The reaction mixture was evaporated and the residue was taken up in 2,2'-
oxybispropane. The resulting solution was washed successively once with a
dilute sodium hydroxide solution and 3 times with water, dried, filtered and
evaporated. The residue was distilled, yielding 2-(bromomethyl)-2-(2,4-
dichlorophenyl)-1,3-dioxolane.
6.9 parts of 1H-1,2,4-triazole in 150 parts of dimethylformamide were added
to a stirred solution of 2.3 parts of sodium in 120 parts of methanol. The
methanol was removed at normal pressure until the internal temperature of
130°C was reached. Then, 25 parts of 2-(bromomethyl)-2-(2,4-
dichlorophenyl)-1,3-dioxolane were added. The reaction-mixture was stirred
and refluxed for 3 h. It was allowed to cool to room temperature and poured
onto water. The precipitated product was filtered off, yielding 12 parts of 1-[2-
(2,4-dichlorophenyl)-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole, melting
point 109.9°C (crystallized from diisopropylether, activated charcoal).
Therapeutic Function
Antifungal
Metabolic pathway
Little published dormation is available on the metabolism of azaconazole.
Degradation
Azaconazole is stable to light under normal storage conditions (but not in ketonic solvents). It is stable at temperatures up to 220 °C.
Properties of Azaconazole
| Melting point: | 109.9° |
| Boiling point: | 460.7±55.0 °C(Predicted) |
| Density | 1.51±0.1 g/cm3(Predicted) |
| vapor pressure | 8.6 x l0-6 Pa (20 °C) |
| storage temp. | 0-6°C |
| pka | 2.94±0.12(Predicted) |
| form | neat |
| Water Solubility | 300 mg l-1 (20 °C) |
| CAS DataBase Reference | 60207-31-0(CAS DataBase Reference) |
| EPA Substance Registry System | Azaconazole (60207-31-0) |
Safety information for Azaconazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Azaconazole
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran


You may like
-
 Azaconazole CAS 60207-31-0View Details
Azaconazole CAS 60207-31-0View Details
60207-31-0 -
 Azaconazole CAS 60207-31-0View Details
Azaconazole CAS 60207-31-0View Details
60207-31-0 -
 Azaconazole CAS 60207-31-0View Details
Azaconazole CAS 60207-31-0View Details
60207-31-0 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
