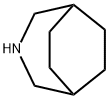
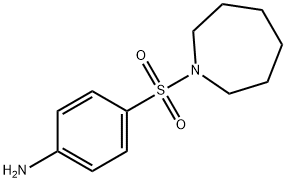
Azabon
- CAS NO.:1150-20-5
- Molecular Weight: 0
- MDL number: MFCD00868089
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-19 05:41:50
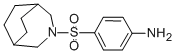
What is Azabon?
Originator
Azabon,ZYF Pharm Chemical
The Uses of Azabon
Stimulant (central).
Definition
ChEBI: Azabon is a member of benzenes and a sulfonamide.
Manufacturing Process
The 1,4-cyclohexane-bis(methylamine) feed line was connected to the top of
the preheater in a pyrolysis tube. The pyrolysis section was heated by
external electric heaters. For a typical run, the pyrolysis temperature was maintained at 385°-395°C, the feed rate was 8.4 g per minute of 1,4-
cyclohexanebis(methylamine) and 1.47 g per minute of nitrogen this is a ratio
of 0.885 mole of nitrogen per mole of 1,4-cyclohexanebis(methylamine) per h.
The crude reaction product was collected in a chilled flask and the nitrogen
and reaction gases were vented to a hood. These conditions were maintained
for 15.5 h, during which time 7,849 g (55 moles) of 1,4-
cyclohexanebis(methylamine) was fed to the unit. Tile total crude product
obtained weighed 7,269 g. Distillation of the crude product at atmospheric
pressure to a base temperature of 260°-270°C yielded 1,480 g of 3-
azabicyclo[3.2.2]nonane (recrystallized from an equal weight of acetone). Gas
chromatography of the filtrates of this first fraction through a 6-foot column
packed with 15% Carbowax 20M on white Chromosorb indicated 1,854 g of
1,4-cyclohexanebis(metnylamine) and 721.0 g of 3-azabicyclo[3.2.2]nonane
present. The remainder of the crude product was distilled at 1-5 mm to a
base temperature of 200°-225°C. Thus, a total of 2,201 g (17.6 moles, 62.1%
yield) of 3-azabicycla[3.2.2]nonane was produced.
To a 3 L, three-neck flask equipped with a stirrer, thremometer, and condenser
was charge 30.0 g (0.24 mole) of 3-azabicyclo[3.2.2]nonane, 44.3 g (0.2
mole) of p-nitrobenzenesulfonyl chloride and 2 L of water. The pH of the
reaction mixture was adjusted to 14 with a 10% solution of sodium hydroxide,
the reaction mixture was then slowly heated to 75°C and heating stopped.
The reaction mixture was cooled to 20°C and 44.0 g (71% of theory) of crude
3-(p-nitrobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane was collected by
filtration.
The crude 3-(p-nitrobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane (44.0 g, 0.14
mole), 5.0 g alcohol wet Raney nickel, and 400 ml methyl alcohol were
charged to an autoclave and reduced at 70°C at 1000 p.s.i. hydrogen
pressure until absorption of hydrogen had stopped. The crude reduced product
was filtered hot to remove the Raney nickel catalyst. The filtrate was cooled to
20°C and the solid 3-(p-ammobenzenesulfonyl)-3-azabicyclo[3.2.2]nonane
was collected by filtration (38.9 g 98% theory), melting point 149°-151°C.
Therapeutic Function
Central stimulant
Safety information for Azabon
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8