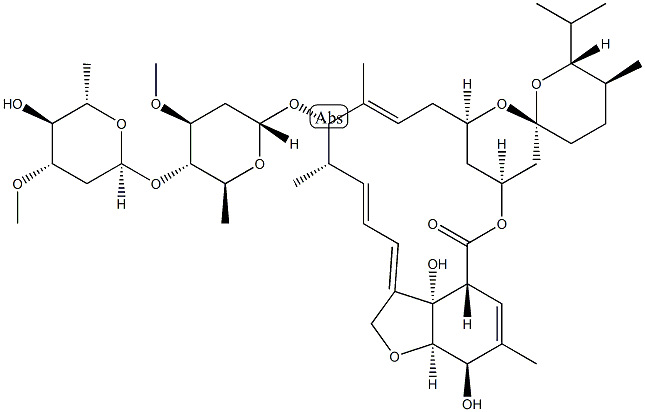
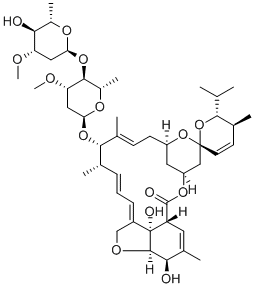
Avermectin A1a, 5-O-demethyl-22,23-dihydro-
- CAS NO.:71827-03-7
- Empirical Formula: C48H74O14
- Molecular Weight: 875.09
- EINECS: 276-046-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-20 14:53:06
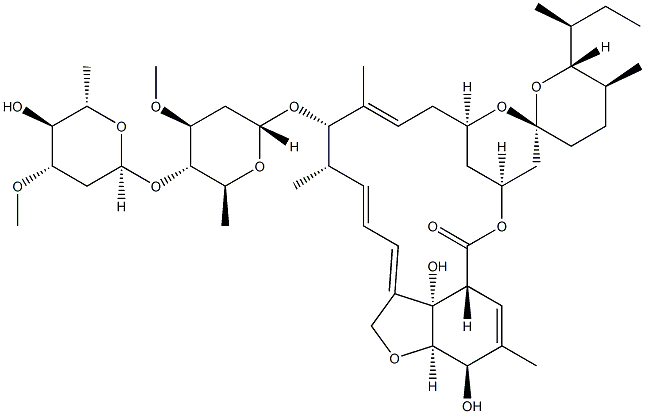
What is Avermectin A1a, 5-O-demethyl-22,23-dihydro-?
Description
Ivermectin B1a is the main component (>80%) of the anthelmintic ivermectin, which also contains ivermectin B1b (<20%; ). It produces antiparasitic activity by binding to glutamate-gated chloride channels expressed on nematode neurons and pharyngeal muscle cells, inducing irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function (EC50 = 104 nM). Formulations containing ivermectin inhibit replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Vero/hSLAM cells.
The Uses of Avermectin A1a, 5-O-demethyl-22,23-dihydro-
Ivermectin B1a-D2 is a deuterium labelled analogue of Ivermectin B1a (I940815), which is a major component of Ivermectin (I940800), a semi-synthetic derivative of Abamectin; consists of a mixture of not less than 80% component B1a and not more than 20% component B1b. Antihelmintic (Onchocerca). An invitro inhibitor of SARS-CoV-2/ Covid-19.
Definition
ChEBI: A macrocyclic lactone that is avermectin B1a in which the double bond present in the spirocyclic ring system has been reduced to a single bond. It is the major component of ivermectin.
References
[1] j. wolsetnholme and a. t. rogers. glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. parasitology131 suppl, s85-s95 (2005).
[2] s. gaisser, l. kellenberger, a. l. kaja, et al. direct production of ivermectin-like drugs after domain exchange in the avermectin polyketide synthase of streptomyces avermitilis atcc31272. organic & biomolecular chemistry 1(16), 2840-2847 (2003).
[3] j. p. arena, k. k. liu, p. s. paress, et al. the mechanism of action of avermectins in caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. journal of parasitology 81, 286-294 (1995).
Properties of Avermectin A1a, 5-O-demethyl-22,23-dihydro-
| Melting point: | 155-157° |
| Boiling point: | 717.97°C (rough estimate) |
| Density | 1.0683 (rough estimate) |
| refractive index | 1.6130 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | solid |
| pka | 12.42±0.70(Predicted) |
| color | white |
Safety information for Avermectin A1a, 5-O-demethyl-22,23-dihydro-
Computed Descriptors for Avermectin A1a, 5-O-demethyl-22,23-dihydro-
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 71827-03-7 Ivermectin 2-epimer (Dihydro Avermectin B1a) 98%View Details
71827-03-7 Ivermectin 2-epimer (Dihydro Avermectin B1a) 98%View Details
71827-03-7 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
