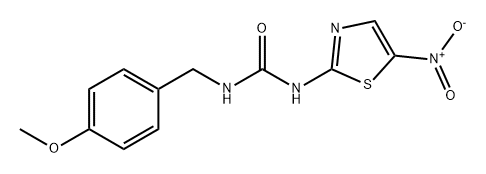
AR-A014418
Synonym(s):AR-A014418, N-(4-Methoxybenzyl)-Nʹ-(5-nitro-1,3-thiazol-2-yl)urea;GSK-3β Inhibitor VIII - CAS 487021-52-3 - Calbiochem
- CAS NO.:487021-52-3
- Empirical Formula: C12H12N4O4S
- Molecular Weight: 308.31
- MDL number: MFCD08277040
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is AR-A014418?
Description
AR-A 014418 (487021-52-3) is a selective glycogen synthase kinase 3 (GSK-3) inhibitor, IC50 = 104 nM. Inhibits β-amyloid-mediated neurodegeneration in hippocampal slices. Anti-inflammatory activity. Cell permeable. Active in rodent models.
Chemical properties
Pale Yellow Solid
The Uses of AR-A014418
AR-A014418 has been used as an inhibitor of glycogen synthase kinase 3 (GSK3).
The Uses of AR-A014418
Glycogen Synthase Kinase 3β (GSK-3β) inhibitor.
What are the applications of Application
GSK-3β Inhibitor VIII is a potent, selective inhibitor of GSK-3β
General Description
A cell-permeable thiazole-containing urea compound that acts as a potent inhibitor of GSK-3. Shown to inhibit GSK-3β with high potency (IC50 = 104 nM). Inhibition is competitive with respect to ATP (Ki = 38 nM). Specificity has been reported using a panel of 28 different kinases, including Cdk2 and Cdk5 (IC50 >100 μM). Shown to prevent tau phosphorylation at a GSK-3-specific site. Also shown to protect neuronal cells against Aβ-mediated neurodegeneration in vitro and reduce tauopathy in mice in vivo (30 μmol/kg delivered with 40% PEG400 and 40% dimethylacetamide in water). Caution: Do not use delivering vehicles containing dimethylamine for in vivo studies, as it is highly toxic to animals. A 25 mM (5 mg/649 μl) solution of GSK-3β Inhibitor VIII (Cat. No. 361557) in DMSO is also available.
Biochem/physiol Actions
Glycogen synthase kinase 3 (GSK3) is a serine/threonine kinase that has been implicated in pathological conditions such as diabetes and Alzheimer′s disease. GSK3 inhibitor, AR-A014418, inhibits GSK3 (IC50 = 104 nM), in an ATP-competitive manner (Ki = 38 nM). AR-A014418 does not significantly inhibit cdk2 or cdk5 (IC50 > 100 μM) or 26 other kinases, demonstrating high specificity for GSK3.
in vitro
ara014418 acted as an atp-competitive manner and did not significantly inhibit cdk2 or cdk5 or 26 other kinases demonstrating high specificity for gsk3. ar-a014418 inhibited tau phosphorylation at a gsk3-specific site (ser-396) in cells stably expressing human four-repeat tau protein [1].
Enzyme inhibitor
This ATP-competitive protein kinase inhibitor (FW = 308.31 g/mol; CAS 487021-52-3; Solubility: 62 mg/mL DMSO; <1 mg/mL H2O) selectively targets glycogen synthase kinase 3β (GSK3β),with IC50 = 104 nM; Ki = 38 nM. Attesting to its high specificity for GSK3, AR-A014418 does not significantly inhibit Cdk2 or Cdk5 (IC50 > 100 μM) or 26 other kinases. AR-A014418 inhibits phosphorylation of the microtubule-associated protein Tau at Ser-396 (i.e., the GSK3-specific site) in cells stably expressing human four-repeat Tau. AR-A014418 protects N2A neuroblastoma cells against cell death mediated by inhibition of the phosphatidylinositol 3-kinase/protein kinase B survival pathway. AR- A014418 inhibits neurodegeneration mediated by b-amyloid peptide in hippocampal slices. Treatment of neuroblastoma cell lines with AR- A014418 reduced the level of GSK-3α phosphorylation at Tyr-279 compared to GSK-3β phosphorylation at Tyr-216, and attenuated growth via the maintenance of apoptosis.. AR-A014418-dependent antinociceptive effects are induced by modulation of the glutamatergic system through metabotropic and ionotropic (NMDA) receptors and the inhibition of TNF-α and IL-1β cytokine signaling.
in vivo
ara014418 induced behavioural changes that were consistent with the effects of antidepressant drugs. subacute intraperitoneal injections of ar-a014418 reduced immobility time in rats exposed to the forced swim test, which was a well-established model for antidepressant efficacy. moreover, the specificity of this effect was supported by our finding that ar-a014418 decreased spontaneous as well as amphetamine-induced activity [2].
Storage
+4°C
References
1) Bhat et al. (2003), Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418; J. Biol. Chem., 278 45937 2) Ramirez et al. (2010), Inhibition of glycogen synthase kinase 3beta (GSK3β) decreases inflammatory responses in brain endothelial cells; Am. J. Pathol., 176 881
Properties of AR-A014418
| Melting point: | 208-210?C (dec.) |
| Density | 1.464±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL, clear, light yellow |
| pka | 5.80±0.70(Predicted) |
| form | Yellow solid |
| color | off-white to tan |
| Sensitive | Light Sensitive |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
Safety information for AR-A014418
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for AR-A014418
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![2-THIO(3-IODOBENZYL)-5-(1-PYRIDYL)-[1,3,4]-OXADIAZOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB2201711.gif)
You may like
-
 GSK-3β Inhibitor VIII CAS 487021-52-3View Details
GSK-3β Inhibitor VIII CAS 487021-52-3View Details
487021-52-3 -
 AR-A014418 CAS 487021-52-3View Details
AR-A014418 CAS 487021-52-3View Details
487021-52-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
