Anirolac
- CAS NO.:66635-85-6
- Empirical Formula: C16H15NO4
- Molecular Weight: 285.29
- MDL number: MFCD00866124
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
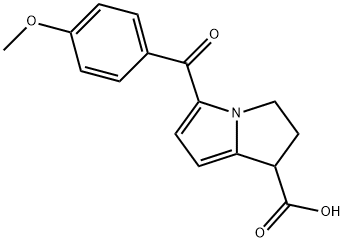
What is Anirolac?
Originator
Anirolac,Syntex Inc.
The Uses of Anirolac
Anirolac is a nonsteroidal anti-inflammatory drug with analgesic properties. Study suggests that pain relief due to Anirolac is equivalent to that of Naproxen (N377520).
The Uses of Anirolac
Antiinflammatory; analgesic.
Manufacturing Process
A solution of 1.1 equivalent of N,N-dimethyl-p-methoxybenzamide and 1 equivalent of phosphorous oxychloride in 2 ml of 1,2-dichloroethane is
refluxed for 30 minutes. To this solution is added a solution of 1 equivalent of
isopropyl 1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate in 2 ml of 1,2-
dichloroethane. The reaction mixture is refluxed under an argon atmosphere
for 8 hours, treated with equivalent of sodium acetate and refluxed for a
further 5 hours. The resultant mixture is then evaporated to dryness and the
residue is chromatographed on 12 g of silica gel, eluting with hexane: ethyl
acetate (3:1), monitoring the course of the reaction by TLC. Isopropyl 5-pmethoxybenzoyl-
1,2-dihydro-3H-pyrrolo[1,2-a]pyrrole-1-carboxylate was
obtained as an oil. UV, IR, NMR spectrum confirmed the structure of obtained
compound.
A solution of 1 equivalent of isopropyl 5-p-methoxybenzoyl-1,2-dihydro-3Hpyrrolo[
1,2-a]pyrrole-1-carboxylate in 10 ml of methanol is treated with a
solution of 1 equivalent of potassium carbonate in 5 ml of water. The reaction
mixture is refluxed under nitrogen atmosphere for 30 minutes, cooled, and
evaporated to dryness. The residue is taken up in 10 ml of 10% aqueous
hydrochloric acid and 50 ml of water and the resultant mixture extracted with
ethyl acetate (2x50 ml). The combined extracts are dried over magnesium
sulfate and evaporated to dryness under reduced pressure. Crystallization of
the residue from ethyl acetate-hexane affords 5-p-methoxybenzoyl-1,2-
dihydro-3H-pyrrolo[1, 2-a]pyrrole-1-carboxylic acid (anirolac); MP: 187°-
187.5°C.
Therapeutic Function
Antiinflammatory, Analgesic
Properties of Anirolac
| Boiling point: | 532.6±50.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| pka | 4.27±0.20(Predicted) |
Safety information for Anirolac
Computed Descriptors for Anirolac
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran

You may like
-
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 Tetrabutylammonium perchlorate 98+View Details
1923-70-2 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
