Aluminum chlorohydrate
- CAS NO.:1327-41-9
- Empirical Formula: AlClHO
- Molecular Weight: 79.44
- MDL number: MFCD00143587
- EINECS: 215-477-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-31 14:38:37
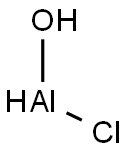
What is Aluminum chlorohydrate?
Chemical properties
often supplied as a suspension or solution in water
The Uses of Aluminum chlorohydrate
In antiperspirants.
Definition
An ingredient of commercial antiperspirant and deodorant preparations. Also used for water purification and treatment of sewage and plant effluent.
General Description
A clear amber liquid with little or no odor. Denser than water. May severely irritate skin, eyes or mucous membrane. Used in commercial antiperspirants and deodorants.
Air & Water Reactions
Will be soluble in water with release of heat.
Reactivity Profile
Aluminum chlorohydrate reacts to neutralize chemical bases (for example: amines and inorganic hydroxides) Reacts with or corrodes active metals, including such structural metals as aluminum and iron, to release hydrogen, a flammable gas. Can initiate the polymerization of certain alkenes. Reacts with cyanide compounds to release gaseous hydrogen cyanide. May generate flammable and/or toxic gases in contact with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and strong reducing agents. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (to give SO2), and carbonates (to give CO2).
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Not classified
Properties of Aluminum chlorohydrate
| Density | 1.36[at 20℃] |
| vapor pressure | 0.001Pa at 20℃ |
| solubility | soluble in H2O |
| form | glassy solid |
| color | glass, glassy solid |
| Water Solubility | soluble H2O, forms slightly turbid colloidal solution [MER06] |
| Stability: | Stable. Incompatible with many metals. |
| InChI | InChI=1S/Al.ClH.H2O.H/h;1H;1H2;/q+2;;;/p-2 |
| CAS DataBase Reference | 1327-41-9(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum chloride hydroxide (1327-41-9) |
Safety information for Aluminum chlorohydrate
Computed Descriptors for Aluminum chlorohydrate
| InChIKey | MTSHNTORJWMHCG-UHFFFAOYSA-L |
| SMILES | [AlH](Cl)O |
Aluminum chlorohydrate manufacturer
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran





You may like
-
 Poly Aluminium Chloride (P.A.C) 99%View Details
Poly Aluminium Chloride (P.A.C) 99%View Details -
 Poly Aluminium Chloride Liquid/Powder 99%View Details
Poly Aluminium Chloride Liquid/Powder 99%View Details -
 Aluminium Chlorohydrate 99%View Details
Aluminium Chlorohydrate 99%View Details -
 aluminum hydroxychloride 98%View Details
aluminum hydroxychloride 98%View Details -
 Aluminium Chlorohydrate (12359-72-7), Purity: 93-95%, Grade: TechnicalView Details
Aluminium Chlorohydrate (12359-72-7), Purity: 93-95%, Grade: TechnicalView Details
12359-72-7 -
 Aluminium ChlorohydrateView Details
Aluminium ChlorohydrateView Details
12042-91-0 -
 Colorless to light yellow Aluminium Chlorohydrate ACH Liquid, Purity: 50 % And 99 %View Details
Colorless to light yellow Aluminium Chlorohydrate ACH Liquid, Purity: 50 % And 99 %View Details
12042-91-0 -
 Aluminium ChlorohydrateView Details
Aluminium ChlorohydrateView Details
12042-91-0
