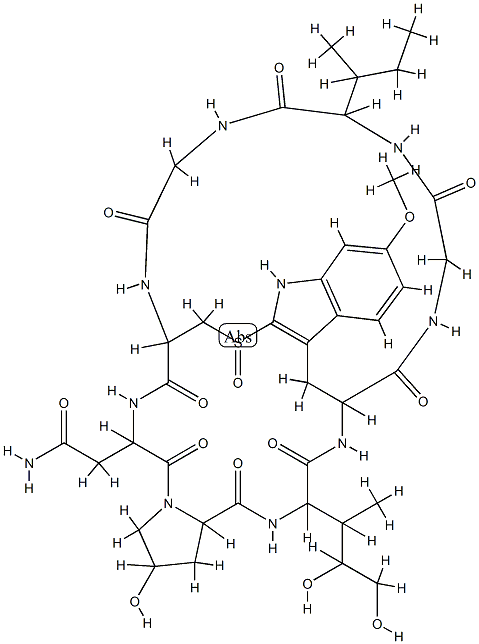
ALPHA-AMANITIN
- CAS NO.:23109-05-9
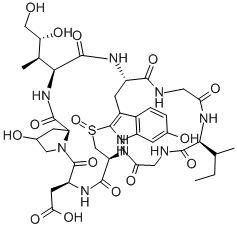
- Empirical Formula: C39H54N10O14S
- Molecular Weight: 918.98
- MDL number: MFCD30207890
- EINECS: 245-432-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:18:54
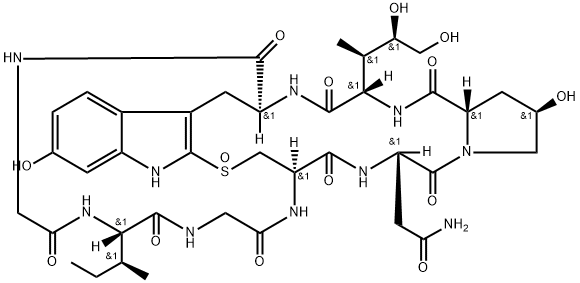
What is ALPHA-AMANITIN?
Description
α-Amanitin, along with its relatives β-, γ-, and ε-amanitin, are highly toxic cyclic peptides produced by species of the mushroom genus Amanita. The most notorious of these fungi is the so-called “death cap”, Amanita phalloides. α-Amanitin is considered to be the deadliest of the death-cap poisons. It has been estimated that A. phalloides accounts for 90% of mushroom-ingested fatalities worldwide.
The amanitins are unusual peptides in that
α-Amanitin’s toxicity derives from its ability to inhibit RNA polymerases II and III. Experiments to establish the chemical nature of the amanitins began in the 1900s, but their structures and properties were not described until the early 1940s by work in the laboratories of Heinrich Otto Wieland (the 1927 chemistry Nobelist) and later his son Theodor*.
Ever since the Wielands’ work, chemists have tried to develop a total synthesis of α-amanitin. This year, it finally happened. David M. Perrin and colleagues at the University of British Columbia (Vancouver) overcame three major impediments to complete the outer ring, the inner ring, and the correct stereochemical placement of the sulfoxide group on the way to producing the molecule.
This synthesis is important because medical researchers require sufficient quantities of α-amanitin to create antibody–drug conjugates made from it. Some of the conjugates have shown promise as anticancer agents.
*The Wielands were quite the chemistry family. Heinrich’s father, also named Theodor, was a pharmaceutical chemist, as was his son Wolfgang. Another son, Otto, was a professor of medicine. His daughter Eva married biochemist Feodor Lynen who won the Nobel Prize in physiology or medicine in 1964.
The Uses of ALPHA-AMANITIN
α-Amanitin has been used:
- for inducing transcriptional arrest in NT2 cells prior to immunofluorescence assay
- to induce nephrotoxicity in mice renal tissues
- to induce and analyse genotoxicity in mice bone marrow cells by cell viability assay, comet assay and chromosomal aberration assay
What are the applications of Application
α-Amanitin is a specific and potent inhibitor of eukaryotic RNA pol II and a mild inhibitor of RNA pol III
Biological Activity
α-amanitin, the most deleterious toxin of a. phalloides to humans, inhibits rna polymerase ii (rnapii), causing hepatic and renal failure.
Biochem/physiol Actions
The major toxic constituent of the mushroom, Amanita phalloides, inhibits eukaryotic RNA polymerase II and III, but does not inhibit RNA polymerase I or bacterial RNA polymerase. Inhibits mammalian protein synthesis.
Storage
Store at -20°C
Properties of ALPHA-AMANITIN
| Melting point: | 254-255 °C(lit.) |
| Boiling point: | 1622.2±65.0 °C(Predicted) |
| alpha | D20 +191° |
| Density | 1.1626 (rough estimate) |
| refractive index | 1.7400 (estimate) |
| storage temp. | −20°C |
| solubility | H2O: 1.0 mg/mL |
| appearance | white to light yellow needles |
| form | powder |
| pka | 9.63±0.70(Predicted) |
| color | white to light yellow |
| Water Solubility | Soluble to 5 mM in ethanol and to 5 mM in water |
| Merck | 13,370 |
| BRN | 1071138 |
Safety information for ALPHA-AMANITIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for ALPHA-AMANITIN
| InChIKey | CIORWBWIBBPXCG-PWKRRBCNNA-N |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 α-Amanitin CAS 23109-05-9View Details
α-Amanitin CAS 23109-05-9View Details
23109-05-9 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
