Adipic acid
Synonym(s):Adipic acid;ADI-PURE high purity adipic acid;ADI-PURE LGA adipic acid;Hexanedioic acid
- CAS NO.:124-04-9
- Empirical Formula: C6H10O4
- Molecular Weight: 146.14
- MDL number: MFCD00004420
- EINECS: 204-673-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-27 08:50:14
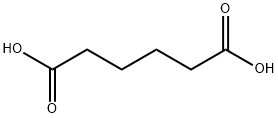
What is Adipic acid?
Description
Adipic acid (butane-1,4-dicarboxylic acid, C6H10O4) is a white crystalline powder of C6-straight chain dicarboxylic acid. It has the lowest acidity of any of the acids commonly used in foods and has excellent buffering capacity in the range of pH 2.5 to 3.0.
Chemical properties
Adipic acid is insoluble in water, soluble in alcohol and acetone. It has low hygroscopicity, long-lasting acidity and high acidity. As a result, adipic acid has found a wide number of uses as an accidulant in dry powdered food mixtures, especially in those products having delicate flavors and where addition of tang to the flavor is undesirable.
Physical properties
Adipic acid is a straight-chain dicarboxylic acid that exists as a white crystalline compound at standard temperature and pressure. Adipic acid is one of the most important industrial chemicals and typically ranks in the top 10 in terms of volume used annually by the chemical industry.
Occurrence
Reported found as a minor constituent in butter, and has been found in other fats as a product of oxidative rancidity. It also occurs in beet juice, pork fat, guava fruit (Psidium guajava L.), papaya (Carica papaya L.) and raspberry (Rubus idaeus L.).
The Uses of Adipic acid
Adipic acid is a dicarboxylic acid that is a useful synthetic reagent. Adipic acid is mainly used in the food industry as an acidifier, buffer, gelling aid, antimicrobial, antioxidant and chelating agent. Adipic acid is also used in the manufacture of plasticisers for polyurethane systems, as a lubricant component and in polyester polyols. It is used in powdered drinks, beverages, gelatin desserts, loz- enges, and canned vegetables. it is also used as a leavening acidulant in baking powder. it can be used as a buffering agent to maintain acidities within a range of ph 2.5–3.0. it is occasionally used in edi- ble oils to prevent rancidity.
Definition
ChEBI: Adipic acid is an alpha,omega-dicarboxylic acid that is the 1,4-dicarboxy derivative of butane. It has a role as a food acidity regulator and a human xenobiotic metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of an adipate(1-).
Production Methods
Adipic acid can be manufactured using several methods, but the traditional and main route of preparation is by the two-step oxidation of cyclohexane (C6H12). In the first step, cyclohexane is oxidized to cyclohexanone and cyclohexanol with oxygen or air. This occurs at a temperature of approximately 150°C in the presence of cobalt or manganese catalysts. The second oxidation is done with nitric acid and air using copper or vanadium catalysts. In this step, the ring structure is opened and adipic acid and nitrous oxide are formed. Other feedstocks such as benzene and phenol may be use to synthesize adipic acid. Adipic acid production used to be a large emitter of nitrous oxide, a greenhouse gas, but these have been controlled in recent years using pollution abatement technology.
Reactions
Adipic acid is a dibasic acid (can be deprotonated twice). Its pKa's are 4.41 and 5.41.
With the carboxylate groups separated by four methylene groups, adipic acid is suited for intramolecular condensation reactions. Upon treatment with barium hydroxide at elevated temperatures, it undergoes ketonization to give cyclopentanone.
Air & Water Reactions
Dust may form explosive mixture with air [USCG, 1999]. Insoluble in water.
Reactivity Profile
Adipic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Adipic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. Behavior in Fire: Melts and may decompose to give volatile acidic vapors of valeric acid and other substances.
Health Hazard
Exposures to adipic acid cause pain, redness of the skin and eyes, tearing or lacrimation. Adipic acid has been reported as a non-toxic chemical. Excessive concentrations of adipic acid dust are known to cause moderate eye irritation, irritation to the skin, and dermatitis.It may be harmful if swallowed or inhaled. It causes respiratory tract irritation with symptoms of coughing, sneezing, and blood-tinged mucous.
Pharmaceutical Applications
Adipic acid is used as an acidifying and buffering agent in
intramuscular, intravenous and vaginal formulations. It is also
used in food products as a leavening, pH-controlling, or flavoring
agent.
Adipic acid has been incorporated into controlled-release
formulation matrix tablets to obtain pH-independent release for
both weakly basicand weakly acidic drugs.It has also been
incorporated into the polymeric coating of hydrophilic monolithic
systems to modulate the intragel pH, resulting in zero-order release
of a hydrophilic drug.The disintegration at intestinal pH of the
enteric polymer shellac has been reported to improve when adipic
acid was used as a pore-forming agent without affecting release in
the acidic media.Other controlled-release formulations have
included adipic acid with the intention of obtaining a late-burst
release profile.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by other routes. A severe eye irritant. Combustible when exposed to heat or flame; can react with oxidzing materials. When heated to decomposition it emits acrid smoke and fumes.
Toxicity
Adipic acid is used in pharmaceutical formulations and food
products. The pure form of adipic acid is toxic by the IP route, and
moderately toxic by other routes. It is a severe eye irritant, and may
cause occupational asthma.
LD50 (mouse, IP): 0.28 g/kg
LD50 (mouse, IV): 0.68 g/kg
LD50 (mouse, oral): 1.9 g/kg
LD50 (rat, IP): 0.28 g/kg
LD50 (rat, oral): >11 g/kg
Potential Exposure
Workers in manufacture of nylon, plasticizers, urethanes, adhesives, and food additives
Storage
Adipic acid is normally stable but decomposes above boiling point. It should be stored in a tightly closed container in a cool, dry place, and should be kept away from heat, sparks, and open flame.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required
Purification Methods
For use as a volumetric standard, adipic acid is crystallised once from hot water with the addition of a little animal charcoal, dried at 120o for 2hours, then recrystallised from acetone and again dried at 120o for 2hours. Other purification procedures include crystallisation from ethyl acetate and from acetone/petroleum ether, fusion followed by filtration and crystallisation from the melt, and preliminary distillation under vacuum. [Beilstein 2 IV 1956.]
Incompatibilities
Adipic acid is incompatible with strong oxidizing agents as well as strong bases and reducing agents. Contact with alcohols, glycols, aldehydes, epoxides, or other polymerizing compounds can result in violent reactions.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed
Precautions
Occupational workers should avoid contact of the adipic acid with the eyes, avoid breathing dust, and keep the container closed. Workers should use adipic acid only with adequate ventilation. Workers should wash thoroughly after handling adipic acid and keep away from heat, sparks, and flame. Also, workers should use rubber gloves and laboratory coats, aprons, or coveralls, and avoid creating a dust cloud when handling, transferring, and cleaning up.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (IM, IV, and vaginal preparations). Accepted for use as a food additive in Europe. Included in an oral pastille formulation available in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Adipic acid
| Melting point: | 151-154 °C(lit.) |
| Boiling point: | 265 °C100 mm Hg(lit.) |
| Density | 1,36 g/cm3 |
| vapor density | 5 (vs air) |
| vapor pressure | 1 mm Hg ( 159.5 °C) |
| refractive index | 1.4880 |
| FEMA | 2011 | ADIPIC ACID |
| Flash point: | 385 °F |
| storage temp. | Store below +30°C. |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| form | Solid |
| pka | 4.43(at 25℃) |
| color | White |
| PH | 3.74(1 mM solution);3.22(10 mM solution);2.71(100 mM solution); |
| Odor | wh. monoclinic prisms, pract. odorless |
| Water Solubility | 1.44 g/100 mL (15 ºC) |
| Merck | 14,162 |
| JECFA Number | 623 |
| BRN | 1209788 |
| Exposure limits | ACGIH: TWA 5 mg/m3 |
| Dielectric constant | 1.8(Ambient) |
| Stability: | Stable. Substances to be avoided include ammonia, strong oxidizing agents. |
| CAS DataBase Reference | 124-04-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Hexanedioic acid(124-04-9) |
| EPA Substance Registry System | Adipic acid (124-04-9) |
Safety information for Adipic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Adipic acid
| InChIKey | WNLRTRBMVRJNCN-UHFFFAOYSA-N |
Adipic acid manufacturer
New Products
Cyclopropane-1,1-dicarboxylic acid Cyclopentane-1,2-dione 2-Bromo-5-iodopyridine 4-isothiocyanato-2-(trifluoroMethyl)benzonitrile Amino-4-methoxyben-zeneacetic acid 3-Aminophenylacetic acid 1-AMino-cyclobutaneMethanol HCl (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid Cefuroxime EP Impurity-A N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 4-Bromo Benzylcyanide 5-azidovalericacid 4-bromo-1H-pyrazole-3-carboxylic acid Isopropyl amine Hydrochloride tert-butyl 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-vinyl-7,8-dihydropyrido[2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)piperazine-1-carboxylateRelated products of tetrahydrofuran



You may like
-
 Adipic Acid 98%View Details
Adipic Acid 98%View Details -
 Adipic acid 98%View Details
Adipic acid 98%View Details -
 ADIPIC ACID. 99%View Details
ADIPIC ACID. 99%View Details -
 Adipic Acid 98%View Details
Adipic Acid 98%View Details -
 ADIPIC ACID 99%View Details
ADIPIC ACID 99%View Details -
 Adipic acid 99%View Details
Adipic acid 99%View Details -
 Adipic Acid CASView Details
Adipic Acid CASView Details -
 Adipic Acid CASView Details
Adipic Acid CASView Details
