Acrylamide
Synonym(s):2-Propenamide;Acrylamide;Acrylic acid amide;Prop-2-enamide
- CAS NO.:79-06-1
- Empirical Formula: C3H5NO
- Molecular Weight: 71.08
- MDL number: MFCD00008032
- EINECS: 201-173-7
- Update Date: 2026-02-10 11:39:28
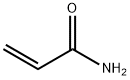
What is Acrylamide?
Description
Acrylamide is the simplest unsaturated organic amide. Despite its low molar mass, and because it is highly polar, it is a solid rather than a liquid. As the hazard information table indicates, it is extremely toxic in multiple ways.
In 1949, Otto Bayer at Bayer AG (Leverkusen, West Germany) described the preparation of acrylamide. (Bayer was not related to the founding Bayer family.) Chemist Bayer synthesized acrylamide via the acid-catalyzed hydrolysis of acrylonitrile. This is the only manufacturing method in use today. But in 2019, Kemira Ltd. (Helsinki, Finland) announced that it will begin to produce biobased acrylamide in its Mobile, AL, plant.
By far the major use of acrylamide is polymerization into polyacrylamides, which, depending on their molar masses and degree of cross-linking, are valuable for flocculating solids suspended in water, thickening water for use in enhanced petroleum recovery, and agricultural soil conditioning.
Acrylamide has become notorious in the past 20 years because traces of it have been found in commercial and home-cooked foods. Most of these are starchy foods such as French fries, potato chips, and some breads. In general, higher temperatures and longer cooking times increase the formation of acrylamide. Smoking tobacco, however, results in much higher blood acrylamide concentrations than any food source.
Chemical properties
Acrylamide, in monomeric form, is an odorless, flake-like crystals which sublime slow at room temperature. May be dissolved in a flammable liquid.
The Uses of Acrylamide
Some of the specific uses of acrylamide are: In liquid-solid separation where acrylamide polymers act as flocculants and aids in mineral processing, waste treatment and water treatment. In the production of polyacrylamides, which are used in water and waste treatment, paper and pulp processing, cosmetic additives, and textile processing; in adhesives and grouts; as cross-linking agents in vinyl polymers.
Definition
acrylamide: An inert gel (polyacrylamide)employed as a medium inelectrophoresis. It is used particularlyin the separation of macromolecules,such as nucleic acids and proteins.
Preparation
The principal synthetic route to making acrylamide involves the hydration of acrylonitrile (ACRN). In this process an aqueous ACRN solution reacts over a copper-oxide-chromium oxide catalyst at approximately 100°C. Several other catalyst systems have been used, and most of them contain copper - in some form. The reaction step is followed by purification and concentration to a 50% solution in a vacuum evaporator. The yield of acrylamide from ACRN is 98%. The purification and concentration steps are costly and also involve the recycle of ACRN back to the reaction step. In the early part of the new century, a catalytic distillation process has been developed that converts almost 100% of the ACRN to acrylamide and allows concentration to occur in the same column where acrylamide is made. Therefore this process is less costly.
Nitto Chemical (now Dia-Nitrix) introduced a biosynthetic route from ACRN to acrylamide in Japan in 1985. This process uses an immobilized nitrile hydratase biocatalyst that converts the ACRN solution to acrylamide with a yield of 99.5%. This high yield allows a concentrated acrylamide solution to be made without the need for ACRN recycle or solution concentration. This process therefore has lower energy costs.
Air & Water Reactions
Acrylamide is very soluble in water. The solvent is not necessarily water soluble.
Reactivity Profile
ACRYLAMIDE SOLUTION reacts with azo and diazo compounds to generate toxic gases. Flammable gases are formed with strong reducing agents. Combustion generates mixed oxides of nitrogen (NOx). Spontaneous, violent polymerization occurs at the melting point (86°C of the undissolved solid [Bretherick, 5th ed., 1995, p. 428].
Health Hazard
The acute toxicity of acrylamide is moderate by ingestion or skin contact. Skin exposure leads to redness and peeling of the skin of the palms. Aqueous acrylamide solutions cause eye irritation; exposure to a 50% solution of acrylamide caused slight corneal injury and slight conjunctival irritation, which healed in 8 days. The chronic toxicity of acrylamide is high. Repeated exposure to ~2 mg/kg per day may result in neurotoxic effects, including unsteadiness, muscle weakness, and numbness in the feet (leading to paralysis of the legs), numbness in the hands, slurred speech, vertigo, and fatigue. Exposure to slightly higher repeated doses in animal studies has induced multisite cancers and reproductive effects, including abortion, reduced fertility, and mutagenicity. Acrylamide is listed in IARC Group 2B ("possible human carcinogen") and is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard.
Flammability and Explosibility
The volatility of acrylamide is low (0.03 mmHg at 40 °C), and it does not pose a significant flammability hazard.
Contact allergens
Acrylamide is used in the plastic polymers industry for water treatments and soil stabilization and to prepare polyacrylamide gels for electrophoresis. This neurotoxic, carcinogenic, and genotoxic substance is known to have caused contact dermatitis in industrial and laboratory workers
Biochem/physiol Actions
Acrylamide is a reactive, water soluble vinyl monomer. It causes a decrease in the number of neuritis per cell as well as reduces the rate of protein synthesis.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and neoplastigenic data. Poison by ingestion, skin contact, and intraperitoneal routes. Experimental reproductive effects. Mutation data reported. A skin and eye irritantIntoxication from it has caused a peripheral neuropathy, erythema, and peeling palms. In industry, intoxication is mainly via dermal route, next via inhalation, and last via ingestion. Time of onset varied from 1-24 months to 8 years. Symptoms were, via dermal route, a numbness, tingling, and touch tenderness. In a couple of weeks, coldness of extremities; later, excessive sweating, bluish-red and peeling palms, marked fatigue and limb weakness. It is dangerous because it can be absorbed through the unbroken skin. From animal experiments it seems to be a central nervous system toxin. Adult rats fed an average of 30 mg/kg for 14 days were all partially paralyzed and had reduced their food consumption by 50 percent. Polymerizes violently at its melting point. When heated to decomposition it emits acrid fumes and NOX,.
Potential Exposure
Added to water during sewage/wastewater treatment. Used in the manufacture of plastics, resins, rubber, synthetic textiles; as a dye, pigment. A major application for monomeric acrylamide is in the production of polymers as polyacrylamides. Polyacrylamides are used for soil stabilization, gel chromatography, electrophoresis, papermaking strengtheners, clarifications, and treatment of potable water and foods.
Carcinogenicity
Acrylamide is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Environmental Fate
Biological. Bridié et al. (1979) reported BOD and COD values of 0.05 and 1.33 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a period of 5 d. When a sewage seed was used in a
separate screening test, a BOD value of 0.92 g/g was obtained. In a treatment plant, a BOD value
of 0.40 g/g was reported after 10 d (Mills et al., 1953). The ThOD for acrylamide is 1.35 g/g.
Soil. Under aerobic conditions, acrylamide degraded to ammonium ions which oxidized to
nitrite ions and nitrate ions. The ammonium ions produced in soil may volatilize as ammonia or
accumulate as nitrite ions in sandy or calcareous soils (Abdelmagid and Tabatabai, 1982).
Chemical/Physical. Readily polymerizes at the melting point or under UV light. In the presence
of alkali, polymerization is a violent reaction. On standing, may turn to yellowish color (Windholz
et al., 1983).
Storage
In particular, this substance should be handled only when wearing appropriate impermeable gloves to prevent skin contact, and all operations that have the potential of producing acrylamide dusts or aerosols of solutions should be conducted in a fume hood to prevent exposure by inhalation.
Purification Methods
Crystallise acrylamide from acetone, chloroform, ethyl acetate, methanol or *benzene/chloroform mixture, then vacuum dry and store it in the dark under vacuum. Recrystallise it from CHCl3 by dissolving 200g in 1L, heating to boiling and filtering without suction in a warmed funnel through Whatman 541 filter paper; allowing to cool to room temperature and keeping at -15o overnight. The crystals are collected with suction in a cooled funnel and washed with 300mL of cold MeOH. The crystals are air-dried in a warm oven. [Dawson et al. Data for Biochemical Research, Oxford Press 1986 p. 449, Beilstein 2 IV 1471.] CAUTION: Acrylamide is extremely TOXIC (neurotoxic), and precautions must be taken to avoid skin contact or inhalation. Use gloves and handle in a well-ventilated fume cupboard.
Toxicity evaluation
All acrylamide in the environment is synthetic, the main source being the release of the monomer residues from polyacrylamide used in water treatment or in industry. Products and compounds containing polyacrylamide can serve as sources of exposure to residues of acrylamide.
Incompatibilities
Acrylamide may decompose with heat and polymerize at temperatures above 84 C, or exposure to light, releasing ammonia gas. Reacts violently with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Reacts with reducing agents; peroxides, acids, bases, and vinyl polymerization initiators. Fine particles of dust form explosive mixture with air.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Acrylamide residue and sorbent material may be packaged in epoxy-lined drums and taken to an EPAapproved disposal site. Incineration with provisions for scrubbing of nitrogen oxides from flue gases. Deep well injection.
Properties of Acrylamide
| Melting point: | 82-86 °C(lit.) |
| Boiling point: | 125 °C25 mm Hg(lit.) |
| Density | 1,322 g/cm3 |
| vapor density | 2.45 (vs air) |
| vapor pressure | 0.03 mm Hg ( 40 °C) |
| refractive index | 1.460 |
| Flash point: | 138 °C |
| storage temp. | 2-8°C |
| solubility | 2040 g/L (25°C) |
| form | powder |
| appearance | white crystals |
| pka | 15.35±0.50(Predicted) |
| color | White |
| Odor | Odorless solid |
| PH | 5.0-7.0 (50g/l, H2O, 20℃) |
| Water Solubility | Acrylamide is routinely tested at 250 mg/mL in water, giving a clear colorless solution. It is soluble at least to 40% (w/v) in water, and reportedly up to 215 g/100 mL in water at 30°C. |
| Sensitive | Light Sensitive |
| Merck | 14,129 |
| BRN | 605349 |
| Henry's Law Constant | (x 10-9 atm?m3/mol):
3.03 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: TWA 0.03, IDLH:
60; OSHA PEL: TWA 0.3; ACGIH TLV: TWA 0.03. |
| Stability: | Unstable. Do not heat above 50C. Explosive. Incompatible with acids, bases, oxidizing agents, reducing agents, iron and iron salts, copper, aluminium, brass, free radical initiators. Air sensitive. Hygroscopic. |
| CAS DataBase Reference | 79-06-1(CAS DataBase Reference) |
| IARC | 2A (Vol. 60, Sup 7) 1994 |
| NIST Chemistry Reference | Acrylamide(79-06-1) |
| EPA Substance Registry System | Acrylamide (79-06-1) |
Safety information for Acrylamide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H340:Germ cell mutagenicity H350:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acrylamide
| InChIKey | HRPVXLWXLXDGHG-UHFFFAOYSA-N |
Acrylamide manufacturer
Gandhi Chemicals
New Products
Tetrabutylammonium perchlorate DL-beta-(3-Bromophenyl)alanine N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile Rifaximin EP Impurity-G N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Diethoxy Benzylcyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran

You may like
-
 Acrylamide 99%View Details
Acrylamide 99%View Details -
 ACRYLAMIDE POWDER 99%View Details
ACRYLAMIDE POWDER 99%View Details -
 ACRYLAMIDE 99%View Details
ACRYLAMIDE 99%View Details -
 Acrylamide CAS 79-06-1View Details
Acrylamide CAS 79-06-1View Details
79-06-1 -
 Acrylamide CAS 79-06-1View Details
Acrylamide CAS 79-06-1View Details
79-06-1 -
 29007 ACRYLAMIDE 99%View Details
29007 ACRYLAMIDE 99%View Details
29007 -
 ACRYLAMIDE CASView Details
ACRYLAMIDE CASView Details -
 Industrial Grade ACRYLAMIDEView Details
Industrial Grade ACRYLAMIDEView Details
79-06-1
