Acetic anhydride
Synonym(s):Acetic Anhydride;Acetyl acetate, Acetyl oxide;Acetyl Oxide,Ethanoic Anhydride, Acetic Acid
- CAS NO.:108-24-7
- Empirical Formula: C4 H6 O3
- Molecular Weight: 102.09
- MDL number: MFCD00008705
- EINECS: 203-564-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:17:03
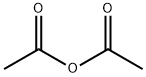
What is Acetic anhydride?
Description
Acetic anhydride (chemical formula: (CH3CO)2O) is the simplest isolable anhydride of a carboxylic acid, is widely used as a reagent in organic synthesis. It has an internal asymmetric structure, leading to its potent electrophilicity. In organic chemistry, it is mainly used in acetylation for the manufacture of commercially significant materials, e.g. it can be used for the conversion of cellulose to cellulose acetate and aspirin. It can also be used as a wood preservative. In starch industry, it is a common acetylation compound for acetylation of monoglyceride. It is also an esterification agent for the production of modified starches. It is produced by carbonylation of methyl acetate or the reaction between ketene and acetic acid.
Description
Acetic anhydride (Ac2O) is a liquid with a strong acetic acid smell (acetic acid is liberated during the hydrolysis of acetic anhydride by moisture in the air). Acetic anhydride is typically used for acetylation of alcohols or amines.
Chemical properties
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis. It is a combustible, colorless, strongly refractive, liquid that smells strongly of acetic acid, formed by its reaction with the moisture in the air. Acetic anhydride is an esterification agent used in the preparation of modified starch and for acetylation of acetylated monoglycerides.
Physical properties
Acetic anhydride is a colorless, very mobile liquid with a very strong, acetic acid-like odor. Experimentally determined detection and recognition odor threshold concentrations were <600 μg/m3 (<140 ppbv) and 1.5 mg/m3 (360 ppbv), respectively (Hellman and Small, 1974). Acetic anhydride is miscible with polar solvents and dissolves in cold alcohol with slow decomposition. The solubility of acetic anhydride in water at 20°C is 2.6wt%, with slow decomposition; the solubility of water in acetic anhydride at 15°C is 10.7 wt%, with gradual decomposition.
Occurrence
Reported found in watercress (Nasturtium officinale r. br.).
The Uses of Acetic anhydride
Acetic anhydride is used chiefly as an acetylating and dehydrating agent; it is used on a large scale for the acetylation of cellulose. Other areas of application for acetic anhydride are:
1) The production of poly(methylacrylimide) hard foam, where acetic anhydride is used for binding the ammonia that is liberated on conversion of two amide groups to an imide group.
2) Acetylated plastic auxiliaries, such as glycerol triacetate, acetyl tributyl citrate, and acetyl ricinolate.
3) Preparation of anhydrous acetic acid in nonaqueous titrimetry.
The Uses of Acetic anhydride
Acetic anhydride is an important solvent and acetylation agent. It is used for the manufacture of acetylcellulose, acetylsalicylic acid, acetanilide, nitrofurane, sulfonamides, vitamin B6 etc. As acetulizer and solvent in examining wool fat, glycerol, fatty and volatile oils, resins; detection of rosin. Widely used in organic syntheses, e.g., as dehydrating agent in nitrations, sulfonations and other reactions where removal of water is necessary.
Definition
ChEBI: Acetic anhydride is an acyclic carboxylic anhydride derived from acetic acid. It has a role as a metabolite and a reagent.
Production Methods
Acetic anhydride is produced by carbonylation of methyl acetate :
CH3CO2CH3 + CO → (CH3CO)2O
This process involves the conversion of methyl acetate to methyl iodide and an acetate salt. Carbonylation of the methyl iodide in turn affords acetyl iodide, which reacts with acetate salts or acetic acid to give the product. Rhodium iodide and lithium iodide are employed as catalysts. Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous conditions. In contrast, the Monsanto acetic acid process, which also involves a rhodium catalyzed carbonylation of methyl iodide, is at least partially aqueous.
To a decreasing extent, acetic anhydride is also prepared by the reaction of ethenone (ketene) with acetic acid at 45–55 °C and low pressure (0.05–0.2 bar).
H2C= C= O + CH3COOH → (CH3CO)2O (ΔH = ?63 kJ/mol)
Ketene is generated by dehydrating acetic acid at 700–750 °C in the presence of triethyl phosphate as a catalyst or (in Switzerland and the CIS) by the thermolysis of acetone at 600–700 °C in the presence of carbon disulfide as a catalyst.
CH3COOH H2C= C= O + H2O (ΔH = +147 kJ/mol)
CH3COCH3 → H2C= C= O + CH4
The route from acetic acid to acetic anhydride via ketene was developed by Wacker Chemie in 1922, when the demand for acetic anhydride increased due to the production of cellulose acetate.
Due to its low cost, acetic anhydride is purchased, not prepared, for use in research laboratories.
What are the applications of Application
As indicated by its organic chemistry, Ac2O is mainly used for acetylations leading to commercially significant materials. Its largest application is for the conversion of cellulose to cellulose acetate, which is a component of photographic film and other coated materials. Similarly it is used in the production of aspirin (acetylsalicylic acid), which is prepared by the acetylation of salicylic acid.It is also used as a wood preservative via autoclave impregnation to make a longer lasting timber.
In starch industry, acetic anydride is a common acetylation compound, used for the production of modified starches
Because of its use for the synthesis of heroin by the diacetylation of morphine, acetic anhydride is listed as a U.S. DEA List II precursor, and restricted in many other countries.
Reactions
introduction of acetyl groups to organic substrates . In these conversions, acetic anhydride is viewed as a source of CH3CO+. Alcohols and amines are readily acetylated. For example, the reaction of acetic anhydride with ethanol yields ethyl acetate:
(CH3CO)2O + CH3CH2OH → CH3CO2CH2CH3 + CH3COOH
Often a base such as pyridine is added to function as catalyst. In specialized applications, Lewis acidic scandium salts have also proven effective catalysts.
Aromatic rings are acetylated, usually in the presence of an acid catalyst. Illustrative is the conversion of benzene to aceto phenone:
(CH3CO)2O + C6H6 → CH3COC6H5 + CH3CO2H
Ferrocene can be acetylated as well:
Cp2Fe + (CH3CO)2O → CpFe(C5H4COCH3).
Aroma threshold values
Detection: 490 ppb; Recognition: 1.5 ppm
Synthesis Reference(s)
Synthetic Communications, 3, p. 63, 1973 DOI: 10.1080/00397917308062006
Tetrahedron Letters, 27, p. 4937, 1986 DOI: 10.1016/S0040-4039(00)85101-1
General Description
A clear colorless liquid with a strong odor of vinegar. Flash point 129°F. Corrosive to metals and tissue. Density 9.0 lb /gal. Manufacture of cellulose esters, plastics, pharmaceuticals, photographic films, cigarette filters, and magnetic tape; inorganic synthesis as an acetylating agent, bleaching agent, and dehydrating agent.
Air & Water Reactions
Flammable. Reacts violently with water to generate acetic acid . This reaction is heightened by the presence of mineral acids (nitric, perchloric, sulfuric acid, etc.) [Chem. Eng. News 25, 3458].
Reactivity Profile
Acetic anhydride reacts violently on contact with water, steam, methanol, ethanol, glycerol and boric acid. Reaction with water is particularly dangerous in presence with mineral acids (e.g., nitric, perchloric, chromic, sulfuric acid) [Chem. Eng. News 25, 3458]. Potentially explosive reactions with oxidizing reagents such as barium peroxide, chromium trioxide, chromic acid, hypochlorous acid, nitric acid, perchloric acid, peroxyacetic acid, potassium permanganate, hydrogen peroxide. [Sax, 9th ed., 1996, p. 15]. Reacts violently with metal nitrates used as nitrating agents [Davey W. et al., Chem. & Ind., 1948, p. 814].
Health Hazard
Liquid is volatile and causes little irritation on uncovered skin. However, causes severe burns when clothing is wet with the chemical or if it enters gloves or shoes. Causes skin and eye burns and irritation of respiratory tract. Nausea and vomiting may develop after exposure.
Flammability and Explosibility
Flammable
Chemical Reactivity
Reactivity with Water: Reacts slowly with water, but considerable heat is liberated when contacted with spray water; Reactivity with Common Materials: Corrodes iron, steel and other metals; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Dilute with water and use sodium bicarbonate solution to rinse; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by inhalation, ingestion, and skin contact. A skin and severe eye irritant. A flammable liquid. A fire and explosion hazard when exposed to heat or flame. Potentially explosive reactions with barium peroxide, boric acid, chromium trioxide, 1,3diphenyltriazene, hydrochloric acid + water, hypochlorous acid, nitric acid, perchloric acid + water, peroxyacetic acid, potassium permanganate, tetrafluoroboric acid, 4toluenesulfonic acid + water, and acetic acid + water. Reactions with ethanol + sodium hydrogen sulfate, and hydrogen peroxide form explosive products. Reactions with ammonium nitrate + hexamethylenetetrammonium acetate + nitric acid form as products the military explosives RDX and HMX. Reacts violently with N-tert-butyl-phthalimic acid + tetrafluoroboric acid, chromic acid, glycerol + phosphoryl chloride, and metal nitrates (e.g., copper or sodium nitrates). Incompatible with 2-aminoethanol, aniline, chlorosulfonic acid, (CrOs + acetic acid), ethylene-diamine, ethyleneimine, glycerol, oleum, HF, permanganates, NaOH, Na2O2, H2SO4, water, N2O2, (glycerol + phosphoryl chloride). When heated to decomposition it emits toxic fumes; can react vigorously with oxidizing materials, wdl react violently on contact with water or steam. Used in production of drugs of abuse. To fight fire, use CO2, dry chemical, water mist, alcohol foam. See also ANHYDRIDES.
Synthesis
It may be synthesized from diacetyl by partial reduction with zinc and acid or may be derived from fermentation
Potential Exposure
Acetic anhydride is used as an acetylating agent or as a solvent in the manufacture of cellulose acetate, acetanilide, aspirin, synthetic fibers, plastics, explosives, resins, perfumes, and flavorings; and it is used in the textile dyeing industry. It is widely used as a pharmaceutical intermediate and as a pesticide intermediate
Environmental Fate
Chemical/Physical. Slowly dissolves in water forming acetic acid. In ethanol, ethyl acetate is formed (Windholz et al., 1983).
Shipping
UN1715 Acetic anhydride, Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid.
Purification Methods
Adequate purification can usually be achieved by fractional distillation through an efficient column. Acetic acid can be removed by prior refluxing with CaC2 or with coarse Mg filings at 80-90o for 5days, or by distillation from a large excess of quinoline (1% AcOH in quinoline) at 75mm pressure. Acetic anhydride can also be dried by standing with Na wire for up to a week, removing the Na and distilling it under vacuum. (Na reacts vigorously with acetic anhydride at 65-70o). Dippy & Evans [J Org Chem 15 451 1950] let the anhydride (500g) stand over P2O5 (50g) for 3hours, then decanted it and stood it with ignited K2CO3 for a further 3hours. The supernatant liquid was distilled and the fraction b 136-138o was further dried with P2O5 for 12hours, followed by shaking with ignited K2CO3, before two further distillations through a five-section Young and Thomas fractionating column. The final material distilled at 137.8-138.0o. It can also be purified by azeotropic distillation with toluene: the azeotrope boils at 100.6o. After removal of the remaining toluene, the anhydride is distilled [sample had a specific conductivity of 5 x 10-9 ohm-1cm -1]. [Beilstein 2 H 96, 2 I 39, 2 II 91, 2 III 134, 2 IV 94.] Rapid procedure: Shake with P2O5, separate, shake with dry K2CO3 and fractionally distil.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alcohols, chromic acid (violent reaction), amines, strong caustics; finely divided metals. Contact with water forms acetic acid and liberates a large amount of heat. Corrosive to iron, steel and other metals.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Acetic anhydride
| Melting point: | -73.1 °C |
| Boiling point: | 140 °C |
| Density | 1.087 |
| vapor density | 3.5 (vs air) |
| vapor pressure | 10 mm Hg ( 36 °C) |
| refractive index | n |
| Flash point: | 130 °F |
| storage temp. | Store at RT. |
| solubility | Miscible with ether, chloroform and benzene. |
| form | Liquid |
| appearance | Colorless liquid |
| color | Colorless |
| Specific Gravity | 1.082 |
| PH | 3 (10g/l, H2O, 20°C) |
| Odor | Very strong; pungent; vinegar-like characteristic odor. |
| explosive limit | 2.0-10.2%(V) |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| Merck | 14,56 |
| BRN | 385737 |
| Exposure limits | NIOSH REL: ceiling 5 ppm (20 mg/m3), IDLH 200 ppm; OSHA PEL: 5 ppm;
ACGIH TLV: ceiling 5 ppm. |
| Dielectric constant | 20.0 |
| Stability: | Stability Flammable. Incompatible with strong oxidizing agents, water, strong bases, alcohols. |
| CAS DataBase Reference | 108-24-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid anhydride(108-24-7) |
| EPA Substance Registry System | Acetic anhydride (108-24-7) |
Safety information for Acetic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetic anhydride
Acetic anhydride manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrile 3,4 Dimethoxy Benzylcyanide 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
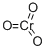


You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8
