6-Maleimidocaproic acid
Synonym(s):MCA;6-Maleimidocaproic acid;Maleimidocaproic acid;N-Maleoyl-6-aminocaproic acid;2,5-Dihydro-2,5-dioxo-1H-pyrrole-1-hexanoic acid
- CAS NO.:55750-53-3
- Empirical Formula: C10H13NO4
- Molecular Weight: 211.21
- MDL number: MFCD00043140
- EINECS: 611-311-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:24:40
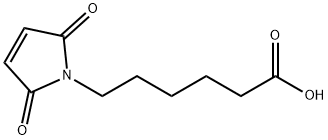
What is 6-Maleimidocaproic acid?
Description
6-Maleimidocaproic acid contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol.
Chemical properties
White to yellow solid, powder, crystals or crystaline powder.
The Uses of 6-Maleimidocaproic acid
6-Maleimidohexanoic acid is used as a probe for introducing maleimides groups into biomolecules and active pharmaceutical ingredients. Further, it is used with N-hydroxysuccinimide ester as a bifunctional cross-linking reagent. In addition to this, it acts as a probe for thiol groups in membrane proteins.
Definition
ChEBI: 6-Maleimidocaproic acid is a medium-chain fatty acid. It contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol.
What are the applications of Application
6-Maleimidocaproic acid is a sulfhydryl reactive heterobifunctional crosslinking reagent. It can be widely used probe for introducing maleimides groups into biomolecules. A probe for thiol groups in proteins. Spacer Arm: 9.4 Angstroms. Probe for thiol groups (SH-groups) in membrane proteins.
Synthesis
Maleic anhydride (29.4 g, 0.3 mol) and 6-aminocaproic acid (39.35 g, 0.3 mol) were refluxed in
glacial acetic acid (900 mL) for 16 h. Acetic anhydride (30.6 g, 0.3 mol) was added dropwise over
a period of 2 hand reflux was continued for 1 h. The acetic acid was removed under vacuum at 70
°C to yield yellow syrup which solidified. The material was chromatographed over silica using
dichloromethane-methanol-acetic acid (100:5:1) affording a crystalline solid (6-Maleimidocaproic acid).
Properties of 6-Maleimidocaproic acid
| Melting point: | 86-91 °C |
| Boiling point: | 407.3±28.0 °C(Predicted) |
| Density | 1.285±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly) |
| pka | 4.74±0.10(Predicted) |
| form | Powder |
| color | White to pale yellow |
| Water Solubility | Soluble in water, methanol, ethanol, dimethyl sulfoxide. Insoluble in ether. |
| Sensitive | Moisture Sensitive |
| BRN | 1532405 |
| CAS DataBase Reference | 55750-53-3(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-hexanoic acid, 2,5-dihydro-2,5-dioxo- (55750-53-3) |
Safety information for 6-Maleimidocaproic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for 6-Maleimidocaproic acid
| InChIKey | WOJKKJKETHYEAC-UHFFFAOYSA-N |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran






You may like
-
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic Acid CAS 55750-53-3View Details
6-Maleimidohexanoic Acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
