5-Benzylidenehydantoin
- CAS NO.:3775-01-7
- Empirical Formula: C10H8N2O2
- Molecular Weight: 188.18
- MDL number: MFCD00014494
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-14 20:44:35
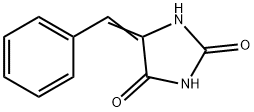
What is 5-Benzylidenehydantoin?
Description
Derivatives of 5-Benzylidenehydantoin are well known of their various attractive bioactivities such as anticancer, antimicrobial, antidiabetic and tyrosinase inhibitor. UPR1024 belongs to a new class of EGFR inhibitors characterized by a hydantoin nucleus and a 5-benzylidene substituent having a double bond conjugated with a carbonyl group on the hydantoin ring. The compound had antiproliferative and proapoptotic effects when tested on the non-small cell lung cancer cell line A549[1-2].
Biological Activity
5-Benzylidenehydantoins inhibited the EGFR kinase and exhibited an antiproliferative action on A431 human epidermoid carcinoma cells. The conjugated exo-cyclic double bond at the C5 position appeared essential for both EGFR and cell growth inhibition, indicating that the 5-benzylidene hydantoin core would be a suitable scaffold for generating new antiproliferative compounds[3].
References
[1] Haq, K. U. et al. “Synthesis of 5-benzylidene-hydantoin and 5-benzylidene-creatinine derivatives under mixed catalyst systems of urea-p-toluenesulfonic acid (Urea-PTSA) and guanidine hydrochloride-triethylamine (GnHCl-TEA).”(2020).
[2] Andrea Cavazzoni. “Dual mechanisms of action of the 5-benzylidene-hydantoin UPR1024 on lung cancer cell lines.” Molecular Cancer Therapeutics 7 2 (2008): 361–70.
Properties of 5-Benzylidenehydantoin
| Melting point: | 220.00°C - 222.00°C |
| Density | 1.329g/cm3 |
| InChI | InChI=1S/C10H8N2O2/c13-9-8(11-10(14)12-9)6-7-4-2-1-3-5-7/h1-6H,(H2,11,12,13,14) |
Safety information for 5-Benzylidenehydantoin
Computed Descriptors for 5-Benzylidenehydantoin
| InChIKey | UDTSPKADQGPZFS-UHFFFAOYSA-N |
| SMILES | C1(=O)NC(=CC2=CC=CC=C2)C(=O)N1 |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran





You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 4-Bromo Benzylcyanide 99%View Details
4-Bromo Benzylcyanide 99%View Details
26451-08-1 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
