Ko 143
Synonym(s):(3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-( 2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4- b]indole-3-propanoic acid 1,1-dimethylethyl ester hydrate;Ko-143 hydrate
- CAS NO.:461054-93-3
- Empirical Formula: C26H35N3O5
- Molecular Weight: 469.57
- MDL number: MFCD11042273
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:25:26
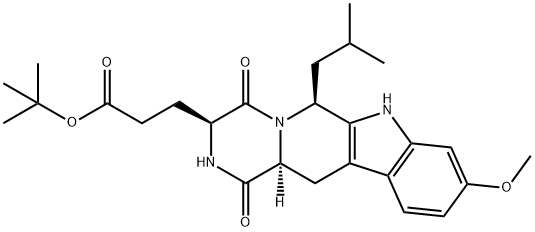
What is Ko 143?
Description
Breast cancer resistance protein (BCRP) is an ATP-
The Uses of Ko 143
A potent BCRP (breast cancer resistance protein) inhibitor
The Uses of Ko 143
Ko143 hydrate has been used:
- to determine the role of ATP-binding cassette sub-family G member 2 (ABCG2), human embryonic kidney (HEK)-C1 and HEK-ABCG2 in tumor microenvironment
- to inhibit ABCG2 for sphere formation assay
- in calcein-AM efflux inhibition to monitor multidrug resistance protein (MRP)-function in kidney
- for cell viability assay
What are the applications of Application
Ko 143 is a selective BCRP multidrug transporter inhibitor
Definition
ChEBI: LSM-6260 is a member of beta-carbolines and a tert-butyl ester.
Biological Activity
Potent and selective breast cancer resistance protein multidrug transporter (BCRP) inhibitor (EC 90 = 26 nM). Displays > 200-fold selectivity over P-gp and MRP-1 transporters. Increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance.
Biochem/physiol Actions
Ko143 has been used as a positive control inhibitor on functions of breast cancer resistance protein (BCRP) using a BCRP prototypical substrate mitoxantrone. BCRP, an ABCG2 transporter, plays an important role in disposition of many drugs and environmental toxins. Ko143 displays > 200-fold selectivity over P-gp and MRP-1 transporters and thus is more specific than other known BCRP inhibitors such as fumitremorgin C and GF120918. It increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance. It blocks topotecan and ABZSO transport in a concentration-dependent manner.
Storage
Store at -20°C
References
1) Allen et al. (2002), Potent and specific inhibition of breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C; Mol. Cancer Ther., 1 417 2) Weidner et al. (2015), The Inhibitor Ko143 Is Not Specific for ABCG2; J. Pharmacol. Exp. Ther., 354 384 3) Palasuberniam et al. (2015), ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy; Sci. Rep., 5 13298 4) Moreno-Sanz et al. (2011), The ABC membrane transporter ABCG2 prevents access of FAAH inhibitor URB937 to the central nervous system; Pharmacol. Res., 64 359 5) Sabnis et al. (2017),?The Efflux Transporter ABCG2 Maintains Prostate Stem Cells;? Mol. Cancer Res., 15 128
Properties of Ko 143
| Melting point: | 147 ºC |
| Boiling point: | 689.8±55.0 °C(Predicted) |
| Density | 1.24 |
| storage temp. | Desiccate at -20°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 13.31±0.60(Predicted) |
| color | white to off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Protect from exposure to moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for Ko 143
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ko 143
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran
You may like
-
 Ko143 hydrate CAS 461054-93-3View Details
Ko143 hydrate CAS 461054-93-3View Details
461054-93-3 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
