3-Maleimidopropionic acid
Synonym(s):N-(2-Carboxyethyl)maleimide;3-Maleimidopropionic acid
- CAS NO.:7423-55-4
- Empirical Formula: C7H7NO4
- Molecular Weight: 169.13
- MDL number: MFCD00043030
- EINECS: 616-069-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:44
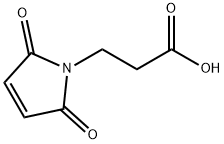
What is 3-Maleimidopropionic acid?
Description
3-Maleimidopropionic acid contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol.
Chemical properties
White to Pale Yellow Solid
The Uses of 3-Maleimidopropionic acid
3-Maleimidopropionic acid is a sulfhydryl reactive heterobifunctional crosslinking reagent.
What are the applications of Application
3-Maleimidopropionic acid is a sulfhydryl reactive heterobifunctional crosslinking reagent
What are the applications of Application
3-Maleimidopropionic acid (3MP) is a molecule that can be used for the treatment of cancer. 3MP has been shown to inhibit the growth of tumor cells and induce apoptosis. It also inhibits the production of reactive oxygen species, which are produced by cancer cells as a result of chemotherapy. 3MP has potent inhibitory activity against bacterial organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium perfringens and antiviral properties against HIV infection. This molecule can also be used in conjunction with monoclonal antibodies or other immunotherapy techniques to improve their efficacy.
General Description
N-Maleoyl-β-alanine (3-maleimidopropanoic acid) is an aliphatic N-substituted maleimide. It is one of the component of the stabilizing solution for EC145 (a folate-targeted vinca alkaloid conjugate) used in rodent pharmacokinetic studies.
Synthesis
An acetic acid solution of maleic anhydride (5.00 g in 50 mL) was added dropwise to an acetic acid solution of β-alanine (4.54 g in 50 mL). The mixture was stirred at room temperature. After 3 h, AcOH (70 mL ) was added and the temperature was raised to 115 °C under stirred overnight. After 1 hour, an orange oil was obtained. The solvent excess was removed under reduced pressure and the product was washed with toluene (3 × 30 mL). The product was then purified by flash chromatography using a silica column (DCM/ethylacetate 9:1) to obtain the white solid 3-Maleimidopropionic acid.
Properties of 3-Maleimidopropionic acid
| Melting point: | 103-106 °C (lit.) |
| Boiling point: | 382.5±25.0 °C(Predicted) |
| Density | 1.473±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.18±0.10(Predicted) |
| color | White |
| Water Solubility | Soluble in methanol, dimethyl formamide, alcohol and dimethyl sulfoxide. Slightly soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 1528952 |
| CAS DataBase Reference | 7423-55-4(CAS DataBase Reference) |
Safety information for 3-Maleimidopropionic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Maleimidopropionic acid
| InChIKey | IUTPJBLLJJNPAJ-UHFFFAOYSA-N |
New Products
Mirtazapine Impurity C/Mirtazapine Lactam Impurity N,O-Dimethylhydroxylamine hydrochloride Tetrabutylammonium perchlorate N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid (R)-1-Benzyl-3-pyrrolidinecarbonitrile N-Nitroso hydroxy Cetrizine EP Impurity-A Noradrenaline EP Impurity D/Noradrenaline Methyl Ether Cetirizine EP Impurity A/Cetirizine CBHP Impurity Lantanoprost rc B Clidinium Bromide Impurity 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 3,4 Diethoxy Benzylcyanide 2-Chloro Benzylcyanide 3-chlorobenzyl cyanide 3,4 Dimethoxy Benzylcyanide valeronitrile 4-Bromo BenzylcyanideRelated products of tetrahydrofuran






You may like
-
 3-Maleimidopropionic Acid CAS 7423-55-4View Details
3-Maleimidopropionic Acid CAS 7423-55-4View Details
7423-55-4 -
 N-Maleoyl-β-alanine CAS 7423-55-4View Details
N-Maleoyl-β-alanine CAS 7423-55-4View Details
7423-55-4 -
 Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
Bendamustine deschloroethyl acid ethyl ester 2517968-40-8 NLT 95%View Details
2517968-40-8 -
 Clidinium Bromide Impurity NLT 95%View Details
Clidinium Bromide Impurity NLT 95%View Details
.6581-06-2 -
 192110-67-2 NLT 95%View Details
192110-67-2 NLT 95%View Details
192110-67-2 -
 Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
Cetirizine EP Impurity A/Cetirizine CBHP Impurity NLT 95%View Details
59872-62-1 -
 .2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 N-Nitroso hydroxy Cetrizine EP Impurity-A NLT 95%View Details
.2005-04-1 -
 145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1 Lantanoprost rc B NLT 95%View Details
145773-22-1
