2,3,4-Trimethoxybenzaldehyde
- CAS NO.:2103-57-3
- Empirical Formula: C10H12O4
- Molecular Weight: 196.2
- MDL number: MFCD00003310
- EINECS: 218-271-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:24:56
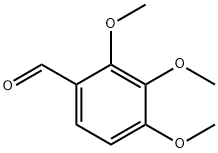
What is 2,3,4-Trimethoxybenzaldehyde?
Description
2,3,4-Trimethoxybenzaldehyde is a white crystalline powder, and the chemical formula is C 10H 12O 4; the molecular weight is 196.2; the fusing point is 38-40 degrees, and is a kind of important medicine intermediate; it is mainly used in new type drug Ca2+ Synthesizing of channel blocker. The alternative cerebral arteries that are used for this medicine reduce the headache incidence, mainly for the preparation of the intermediate of trimetazidine.
Synthesis
A coking gallic acid is utilized as a raw material, and dimethyl sulfate is utilized as an alkylate reagent; under the condition of the existence of sodium hydroxide, methylation is performed through an O-alkylation reaction to obtain an intermediate 1, 2, 3-trimethoxybenzene. After that, the 1,2, 3-trimethoxybenzene and a Vilsmeier-Haack reagent are subjected to a formylation reaction to get the 2,3,4-Trimethoxybenzaldehyde.
Chemical properties
white to light yellow crystals or cryst. powder
Description
2,3,4-Trimethoxybenzaldehyde is white crystalline powder, fusing point is the 38-40 degree, is a kind of important medicine intermediate, is mainly used in newtype drug Ca2+ Synthesizing of channel blocker.
The Uses of 2,3,4-Trimethoxybenzaldehyde
2,3,4-Trimethoxybenzaldehyde is a trimethoxylated aromatic aldehyde. 2,3,4-Trimethoxybenzaldehyde is an Impurity of the anti-anginal drug Trimetazidine (T795610).
The Uses of 2,3,4-Trimethoxybenzaldehyde
2,3,4-Trimethoxybenzaldehyde was used to study the effects of 2,3,4-trimethoxybenzaldehyde on tubulin-dependent GTP hydrolysis.
Preparation
The method includes that a coking gallic acid is utilized as a raw material, dimethyl sulfate is utilized as an alkylate reagent, under the condition of existence of sodium hydroxide, methylation is performed through an O-alkylation reaction to obtain an intermediate 1, 2, 3-trimethoxybenzene, and the 1 ,2, 3-trimethoxybenzene and a Vilsmeier-Haack reagent are subjected to a formylation reaction to obtain the 2, 3, 4-trimethoxybenzaldehyde.
Properties of 2,3,4-Trimethoxybenzaldehyde
| Melting point: | 38-40 °C(lit.) |
| Boiling point: | 168-170 °C12 mm Hg(lit.) |
| Density | 1.2166 (rough estimate) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | methanol: 0.1 g/mL, clear |
| form | Crystals or Crystalline Powder |
| color | White to light yellow |
| Sensitive | Air Sensitive |
| BRN | 981091 |
| InChI | InChI=1S/C10H12O4/c1-12-8-5-4-7(6-11)9(13-2)10(8)14-3/h4-6H,1-3H3 |
| CAS DataBase Reference | 2103-57-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde, 2,3,4-trimethoxy-(2103-57-3) |
| EPA Substance Registry System | Benzaldehyde, 2,3,4-trimethoxy- (2103-57-3) |
Safety information for 2,3,4-Trimethoxybenzaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 2,3,4-Trimethoxybenzaldehyde
| InChIKey | UCTUXUGXIFRVGX-UHFFFAOYSA-N |
| SMILES | C(=O)C1=CC=C(OC)C(OC)=C1OC |
2,3,4-Trimethoxybenzaldehyde manufacturer
JSK Chemicals
ASM Organics
New Products
Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran


You may like
-
 2,3,4 Trimethoxy Benzaldehyde 99%View Details
2,3,4 Trimethoxy Benzaldehyde 99%View Details -
 2103-57-3 98%+View Details
2103-57-3 98%+View Details
2103-57-3 -
 2103-57-3 98%+View Details
2103-57-3 98%+View Details
2103-57-3 -
 2,3,4-Trimethoxybenzaldehyde, 99% CAS 2103-57-3View Details
2,3,4-Trimethoxybenzaldehyde, 99% CAS 2103-57-3View Details
2103-57-3 -
 2,3,4-Trimethoxybenzaldehyde CAS 2103-57-3View Details
2,3,4-Trimethoxybenzaldehyde CAS 2103-57-3View Details
2103-57-3 -
 2,3,4-Trimethoxybenzaldehyde CASView Details
2,3,4-Trimethoxybenzaldehyde CASView Details -
 Trimetazidine EP Impurity CView Details
Trimetazidine EP Impurity CView Details
2103-57-3 -
 2,3,4-TrimethoxybenzaldehydeView Details
2,3,4-TrimethoxybenzaldehydeView Details
2103-57-3
