2-Methyl-1-pentene
- CAS NO.:763-29-1
- Empirical Formula: C6H12
- Molecular Weight: 84.16
- MDL number: MFCD00009405
- EINECS: 212-108-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
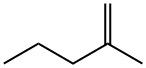
What is 2-Methyl-1-pentene?
Chemical properties
CLEAR COLOURLESS LIQUID
Physical properties
Clear, colorless flammable liquid with an unpleasant hydrocarbon odor.
The Uses of 2-Methyl-1-pentene
Organic synthesis, flavors, perfumes,medicines, dyes, oils, resins.
The Uses of 2-Methyl-1-pentene
2-Methyl-1-pentene is prepared from 2-Methyl-1-pentene. 2-methyl-1-pentene is used in copolymerization of ethylene catalyzed by nonbridged half-titanocenes. The isomerization of 2-methyl-1-pentene leads to anionic activation of c-h bonds in olefins.
Definition
ChEBI: 2-methyl-1-pentene is an alkene that is pent-1-ene carrying a methyl group at position 2. It has a role as a human metabolite. It is an alkene and a volatile organic compound. It derives from a hydride of a pentane.
General Description
Colorless liquid. Flash point -15°F. Floats on water. Irritating vapor.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
2-Methyl-1-pentene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.
Hazard
Flammable, dangerous fire risk.
Health Hazard
INHALATION: May produce anesthetic effects. EYES: Moderate eye irritation possible.
Fire Hazard
Behavior in Fire: Can react vigorously with oxidizing materials.
Source
California Phase II reformulated gasoline contained 2-methyl-1-pentene at a concentration of 1.25 g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic converters were 0.23 and 26.6 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Photolytic. The reported reaction rate constants for the reaction of 2-methyl-1-pentene with OH
radicals and ozone in the atmosphere are 1.05 x 10-17 and 6.26 x 10-11 cm3/molecule?sec,
respectively (Atkinson and Carter, 1984; Atkinson, 1985).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water.
Purification Methods
Water is removed, and formation of peroxides is prevented by several vacuum distillations of 2-methyl-1-pentene from sodium. It is stored with sodium-potassium alloy. [Beilstein 1 IV 841.]
Properties of 2-Methyl-1-pentene
| Melting point: | -136 °C |
| Boiling point: | 62 °C(lit.) |
| Density | 0.682 g/mL at 25 °C(lit.) |
| vapor pressure | 325 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | −15 °F |
| storage temp. | 0-6°C |
| solubility | Soluble in alcohol, benzene, chloroform, petroleum (Weast, 1986); miscible in pentane,
cyclohexene, and other hydrocarbons. |
| form | Liquid |
| color | Clear colorless |
| Water Solubility | Insoluble in water. |
| BRN | 1340499 |
| Henry's Law Constant | 0.28 atm?m3/mol at 25 °C (Mackay and Shiu, 1981) |
| CAS DataBase Reference | 763-29-1(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Methyl-1-pentene (763-29-1) |
Safety information for 2-Methyl-1-pentene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for 2-Methyl-1-pentene
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 2-Methyl-1-pentene CAS 763-29-1View Details
2-Methyl-1-pentene CAS 763-29-1View Details
763-29-1 -
 2-Methyl-1-pentene CAS 763-29-1View Details
2-Methyl-1-pentene CAS 763-29-1View Details
763-29-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
