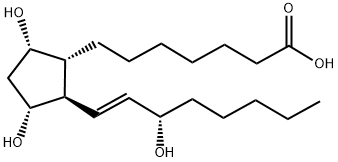
13,14-DIHYDROPROSTAGLANDIN E1
Synonym(s):(9α,11α,13E,15S)-9,11,15-Trihydroxy-6-oxoprosta-13-en-1-oic acid;6-Ketoprostaglandin F1α;6-Oxo-prostaglandin F1α
- CAS NO.:58962-34-8
- Empirical Formula: C20H34O6
- Molecular Weight: 370.48
- MDL number: MFCD00135222
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:13:51
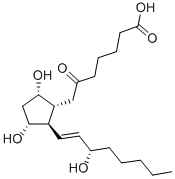
What is 13,14-DIHYDROPROSTAGLANDIN E1?
Description
6-
The Uses of 13,14-DIHYDROPROSTAGLANDIN E1
6-Ketoprostaglandin F1α is the stable hydrolysis product of prostacyclin (P839060), an eicosanoid which prevents the formation of platelet plugs and an effective vasodilator.
What are the applications of Application
6-Keto-prostaglandin F1α is a PGI 2 hydrolysis product used as a marker for PGI 2
Definition
ChEBI: A prostaglandin Falpha that is prostaglandin F1alpha bearing a keto substituent at the 6-position.
Properties of 13,14-DIHYDROPROSTAGLANDIN E1
| Boiling point: | 575.3±50.0 °C(Predicted) |
| Density | 1.191±0.06 g/cm3(Predicted) |
| RTECS | UK8428300 |
| storage temp. | −20°C |
| solubility | ethanol: 10 mg/mL, clear, faintly yellow |
| pka | 4.69±0.10(Predicted) |
| form | White to off-white solid. |
| BRN | 2821925 |
Safety information for 13,14-DIHYDROPROSTAGLANDIN E1
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P321:Specific treatment (see … on this label). P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for 13,14-DIHYDROPROSTAGLANDIN E1
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) 1-aminocyclopentane carbonitrile, HCl H-D-TRP(FOR)-OH HCL 9-Mesityl-10-methylacridinium perchlorate 3-Amino-3-(4-fluorophenyl)propanoic acid Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate Bisacodyl Related Compound C/Bisacodyl EP Impurity C Levothyroxine Beta Hydroxy Impurity Candesartan EP Impurity-F/Candesartan Cilexetil USP Related Compound F / Candesartan Cilexetil N2-Ethyl Impurity / 2H-N2-Ethyl Candesartan Cilexetil Atorvastatin amide impurity/Atorvastatin EP Impurity F(Calcium Salt) Sumatriptan Succinate USP Related Compound C N-[(1S)-1-benzyl-2-({(1R)-3-methyl-1-[(1S,2S,6R,8S)-2,9,9-trimethyl-3,5-dioxa-4-boratricyclo[6.1.1.0~2,6~]dec-4-yl]butyl}amino)-2-oxoethyl]-2-pyrazinecarboxamide N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide L-phenylalanine methyl ester hydrochloride 2,2'-(5-Bromomethyl-1,3-phenylene)-di(2-Methylpropionitrile) tri sodium thio phosphate L-phenylalanine, N-(pyrazinyl carbonyl) methyl ester 3-chlorobenzyl cyanide 2-Chloro Benzylcyanide 3,4 Diethoxy Benzylcyanide 4-Bromo Benzylcyanide 3-Hydroxypropionitrile valeronitrileRelated products of tetrahydrofuran

You may like
-
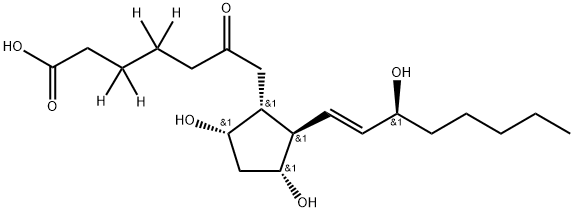
 Epoprostenol Related Compound A CAS 58962-34-8View Details
Epoprostenol Related Compound A CAS 58962-34-8View Details
58962-34-8 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 110-59-8 valeronitrile 99%View Details
110-59-8 valeronitrile 99%View Details
110-59-8 -
 93-17-4 99%View Details
93-17-4 99%View Details
93-17-4 -
 1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 3-chlorobenzyl cyanide 99%View Details
1529-41-5 -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
