Zavegepant
- CAS NO.:1337918-83-8
- Empirical Formula: C36H46N8O3
- Molecular Weight: 638.8
- MDL number: MFCD27938555
- Update Date: 2025-11-07 14:45:54
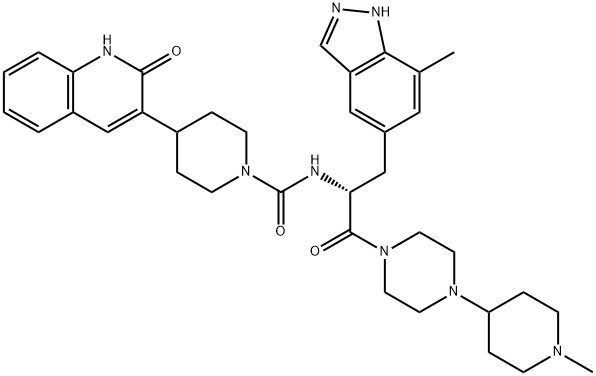
What is Zavegepant?
Absorption
After a single intranasal dose of zavegepant (10 mg), the peak plasma concentration was detected approximately 30 minutes later. The absolute bioavailability of zavegepant administered with a nasal spray is approximately 5%. Up to 40 mg (4 times the recommended dose of 10 mg), a single intranasal dose of zavegepant has slightly less than dose-proportional pharmacokinetics. There was no evidence of zavegepant accumulation with once-a-day zavegepant taken for 14 days.
Compared to normal subjects, patients with moderate hepatic impairment (Child-Pugh B) have a Cmax and AUC 16% and 1.9-fold higher, respectively; however, these changes are not expected to be clinically significant based on clinical safety experience and minimal accumulation of drug exposures. In subjects with estimated creatinine clearance (CLcr) greater or equal to 30 mL/min, the differences in zavegepant pharmacokinetics are not expected to be clinically significant. In patients with a CLcr from 15 to 29 mL/min, zavegepant exposure may increase.
Toxicity
Toxicity information regarding zavegepant is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as serious hypersensitivity reaction occurs. Symptomatic and supportive measures are recommended. The intranasal administration of zavegepant to transgenic mice carrying c-Ha-ras oncogenes (Tg-rasH2) at a maximum concentration of 2.5 mg/day up to 26 weeks did not lead to the development of drug-related tumors. The same results were obtained when rats were given up to 18.8 mg/kg/day of intranasal zavegepant for up to 96 weeks, reaching a plasma exposure 140 times the one in humans receiving the maximum recommended human dose (MRHD). In in vitro and in vivo mutagenesis assays, zavegepant showed negative results. Male and female rats given up to 25 mg/kg/day of zavegepant subcutaneously before and during mating and up to gestation day 7 in females did not present adverse effects on fertility or reproductive performance. Plasma exposure was equivalent to 2800 times the one in humans given the MRHD.
Background
Zavegepant (BHV-3500) is a calcitonin gene-related peptide (CGRP) receptor antagonist. CGRP is released from sensory nerves and acts as a strong vasodilator, and thanks to these properties, it is involved in pain pathways. CGRP receptors are expressed in the central and peripheral nervous system; however, CGRP does not cross the blood-brain barrier, suggesting that it acts on peripheral nerves. In migraine, CGRP innervates pain-producing meningeal blood vessels and is released by trigeminal nerve stimulation. Since they inhibit these mechanisms and desensitize neuronal circuits, the use of CGRP receptor antagonists is beneficial in the treatment of migraine.
Small molecule CGRP antagonists are also known as "gepants", and this category includes other drugs such as rimegepant and ubrogepant. Zavegepant is a third-generation CGRP receptor antagonist that is small in size and highly soluble. Due to its pharmacological properties, it can be administered intranasally. In March 2023, the FDA approved the use of zavegepant nasal spray for the acute treatment of migraine with or without aura in adults. A clinical trial (NCT04804033) is currently investigating the efficacy and safety of oral zavegepant in migraine prevention, and another one (NCT04987944) is evaluating the safety and efficacy of oral zavegepant (150 mg bid) in subjects with mild allergic asthma.
Indications
Zavegepant in a nasal spray form is indicated for the acute treatment of migraine with or without aura in adults. It is not indicated for the preventive treatment of migraine.
Pharmacokinetics
The relationship between the pharmacodynamic activity of zavegepant and its mechanism of action is unclear. No clinically relevant differences were detected when comparing the resting blood pressure of healthy volunteers given sumatriptan and zavegepant concomitantly to those given sumatriptan alone. Using zavegepant leads to a clinically relevant QT interval prolongation at a dose up to 4 times the recommended daily dose. The use of zavegepant may cause hypersensitivity reactions, such as facial swelling and urticaria. If a hypersensitivity reaction occurs, the product label recommends discontinuing zavegepant and initiating appropriate therapy.
Metabolism
In vitro, zavegepant is mainly metabolized by CYP3A4, and by CYP2D6 to a lesser extent. After a single intravenous dose of [14C]-zavegepant (5 mg), approximately 90% of the circulating dose was unchanged zavegepant. None of the zavegepant metabolites detected in plasma were found at a proportion higher than 10% (no major metabolites).
Properties of Zavegepant
| Boiling point: | 933.7±65.0 °C(Predicted) |
| Density | 1.285±0.06 g/cm3(Predicted) |
| pka | 11.68±0.70(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Zavegepant
Computed Descriptors for Zavegepant
Zavegepant manufacturer
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateYou may like
-
 1337918-83-8 98+View Details
1337918-83-8 98+View Details
1337918-83-8 -
 674783-97-2 98+View Details
674783-97-2 98+View Details
674783-97-2 -
 H-D-TRP(FOR)-OH HCL 98+View Details
H-D-TRP(FOR)-OH HCL 98+View Details
367453-01-8 -
 DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
DL-beta-(3-Bromophenyl)alanine 117391-50-1 98+View Details
117391-50-1 -
 49830-37-7 98+View Details
49830-37-7 98+View Details
49830-37-7 -
 3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
3-Amino-3-(4-fluorophenyl)propanoic acid 98+View Details
325-89-3 -
 1428243-26-8 98+View Details
1428243-26-8 98+View Details
1428243-26-8 -
 1-aminocyclopentane carbonitrile, HCl 98+View Details
1-aminocyclopentane carbonitrile, HCl 98+View Details
16195-83-8
