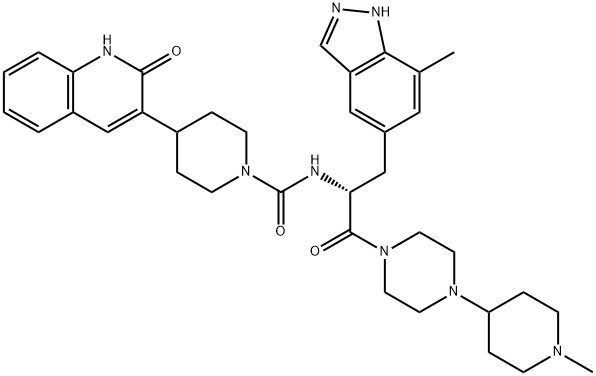
COMPUTED DESCRIPTORS
| Molecular Weight | 638.8 g/mol |
|---|---|
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 638.36928736 g/mol |
| Monoisotopic Mass | 638.36928736 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 1160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Zavegepant is an antagonist of the calcitonin gene-related peptide (CGRP) receptor currently in phase 3 trials in an intranasal formulation for the treatment of migraine. If FDA approved, it will join other previously-approved "-gepant" drugs [rimegepant] and [ubrogepant] as an additional treatment alternative for patients with migraine, particularly those for whom traditional triptan therapy has proven ineffective. On April 15th, 2020, a phase 2 clinical trial (NCT04346615: Safety and Efficacy Trial of Vazegepant Intranasal for Hospitalized Patients With COVID-19 Requiring Supplemental Oxygen) began to investigate the use of intranasally administered zavegepant to combat the acute respiratory distress syndrome (ARDS) sometimes seen in patients with COVID-19. Acute lung injury activates the release of CGRP, which plays a role in the development of ARDS - CGRP antagonists, then, may help to blunt the significant inflammation associated with COVID-19. The clinical trial is expected to complete in September 2020.