1-Iodoadamantane
- CAS NO.:768-93-4
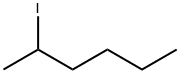
- Empirical Formula: C10H15I
- Molecular Weight: 262.13
- MDL number: MFCD00134444
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:16:17
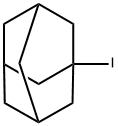
What is 1-Iodoadamantane?
Chemical properties
solid
The Uses of 1-Iodoadamantane
1-Iodoadamantane is suitable reagent used to evaluate the rate constants for the reduction of haloadamantanes by SmI2 in presence of hexamethylphosphoramide (HMPA) and H2O by GC/MS-analyzed method. It may be used as iodine atom donor for probing the intermediacy of radical to investigate the chemistry of highly reactive, strained systems such as propellane. It may be used as starting reagent in the synthesis of N-(1-adamantyl)acetamide via nucleophilic substitution. It may be employed in the free-radical carbonylation reactions with alkenes.
What are the applications of Application
1-Iodoadamantane is used in free-radical carbonylation
Synthesis Reference(s)
The Journal of Organic Chemistry, 48, p. 2766, 1983 DOI: 10.1021/jo00164a026
General Description
1-Iodoadamantane is a haloadamantane. Voltammetric reduction of 1-iodoadamantane at a silver cathode in tetrahydrofuran (THF) and acetonitrile (ACN) is reported to involve a single electron forming a mixture of monomeric and dimeric products. The photoinduced reaction of 1-iodoadamantane in DMSO is reported to afford substitution products on C3, C6, and C8, 1-adamantanol, 1-adamantyl 2-naphthyl ether, and adamantine. The photostimulated reaction of the phthalimide anion with 1-iodoadamantane is reported to yield 3-(1-adamantyl) phthalimide and 4-(1-adamantyl) phthalimide, along with the reduction product adamantane. 1-Iodoadamantane is reported to undergoe photostimulated reaction with the enolate anion of acetone, acetophenone and propiophenone to give admantane and the substitution products.
Purification Methods
Dissolve the iodide in Et2O, shake with aqueous NaHSO3, aqueous K2CO3, and H2O, dry (Na2SO4), evaporate and recrystallise it from MeOH at -70o (to avoid alcoholysis) to give white crystals. [Schleyer & Nicholas J Am Chem Soc 83 2700 1961, lit m of 151-152.5o is incorrect.] Also purify by recrystallisation from pet ether (40-60oC) followed by rigorous drying and repeated sublimation. [Beilstein 5 IV 470.]
Properties of 1-Iodoadamantane
| Melting point: | 75-76 °C(lit.) |
| Boiling point: | 265.7±9.0℃ (760 Torr) |
| Density | 1.63±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.6610 (estimate) |
| Flash point: | 115.9±13.1℃ |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO: 52 mg/mL (198.37 mM);; |
| Stability: | Stable, but light and moisture sensitive. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C10H15I/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6H2 |
Safety information for 1-Iodoadamantane
Computed Descriptors for 1-Iodoadamantane
| InChIKey | PXVOATXCSSPUEM-UHFFFAOYSA-N |
| SMILES | C12(I)CC3CC(CC(C3)C1)C2 |
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
You may like
-
 1-Iodoadamantane CAS 768-93-4View Details
1-Iodoadamantane CAS 768-93-4View Details
768-93-4 -
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
