1-AdaMantanethylaMine
Synonym(s):1-(Aminomethyl)adamantane
- CAS NO.:13392-28-4
- Empirical Formula: C12H21N
- Molecular Weight: 179.3
- MDL number: MFCD00869344
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-02-02 18:10:39
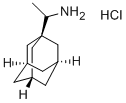
What is 1-AdaMantanethylaMine?
Absorption
Well absorbed, with the tablet and syrup formulations being equally absorbed after oral administration.
Toxicity
Oral LD50 in rats is 640 mg/kg. Overdoses of a related rug, amantadine, have been reported with adverse reactions consisting of agitation, hallucinations, cardiac arrhythmia and death.
The Uses of 1-AdaMantanethylaMine
Rimantadine act by blocking the M2 ion channel which is required for uptake of protons into the interior of the virus to permit acid-promoted viral uncoating (decapsidation).
Indications
For the prophylaxis and treatment of illness caused by various strains of influenza A virus in adults.
Background
An RNA synthesis inhibitor that is used as an antiviral agent in the prophylaxis and treatment of influenza.
Definition
ChEBI: 1-(1-adamantyl)ethanamine is an alkylamine.
brand name
Flumadine (Forest).
General Description
Resistant variants of influenza type A have been recoveredfrom rimantadine-treated patients.Resistance with inhibitory concentrations increased morethan 100-fold have been associated with single nucleotidechanges that lead to amino acid substitutions in the transmembranedomain of M2. rimantadineshare cross-susceptibility and resistance.
Pharmaceutical Applications
An analog of amantadine, supplied as the hydrochloride for oral administration.
Mechanism of action
Rimantadine hydrochloride (α-methyl-1-adamantanemethylamine hydrochloride) is a synthetic adamatane derivative that is structurally and pharmacologically related to amantadine. It appears to be more effective than amantadine hydrochloride against influenza A, with fewer CNS side effects. Rimantadine hydrochloride is thought to interfere with virus uncoating by inhibiting the release of specific proteins. It may act by inhibiting RT or the synthesis of virus-specific RNA, but it does not inhibit virus adsorption or penetration. It appears to produce a virustatic effect early in the virus replication. It is used widely in Russia and Europe.
Pharmacokinetics
Rimantadine, a cyclic amine, is a synthetic antiviral drug and a derivate of adamantane, like a similar drug amantadine. Rimantadine is inhibitory to the in vitro replication of influenza A virus isolates from each of the three antigenic subtypes (H1N1, H2H2 and H3N2) that have been isolated from man. Rimantadine has little or no activity against influenza B virus. Rimantadine does not appear to interfere with the immunogenicity of inactivated influenza A vaccine.
Pharmacokinetics
Oral absorption: >90%
Cmax 100 mg oral (every 12 h): 0.4–0.5 mg/L after 2–6 h
Plasma half-life: c. 35 h
Volume of distribution: Very large
Plasma protein binding: c. 40%
Absorption and distribution
Single- and multiple-dose pharmacokinetic studies in elderly patients and young adults are remarkably similar. The steadystate concentration in nasal mucus develops by day 5 at a concentration approximately 1.5-fold higher than plasma.
Metabolism
In contrast to amantadine, rimantadine is extensively metabolized in the liver by hydroxylation and glucuronidation.
Excretion
Less than 20% is excreted unchanged in the urine and most of the breakdown products are excreted by this route. Thus, the plasma half-life is much less affected by renal dysfunction than that of amantadine.
Clinical Use
Prophylaxis and treatment of influenza A H1N1 infections Since prolonged administration is well tolerated by elderly patients, the drug is preferable to amantadine.
Clinical Use
rimantadine is used for the treatment of diseases caused by influenza A strains.
Side Effects
Rimantadine has significantly fewer side effects than amantadine at equivalent doses, perhaps because of differences in pharmacokinetics, since with equal doses the blood levels are considerably lower. CNS side effects are not significantly higher than placebo.
Metabolism
Following oral administration, rimantadine is extensively metabolized in the liver with less than 25% of the dose excreted in the urine as unchanged drug. Glucuronidation and hydroxylation are the major metabolic pathways.
Properties of 1-AdaMantanethylaMine
| Boiling point: | 248°C |
| Density | 1.033 |
| Flash point: | 99°C |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Liquid |
| pka | 11.17±0.29(Predicted) |
| color | Colorless to light yellow |
| CAS DataBase Reference | 13392-28-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Adamantanemethylamine, «alpha»-methyl-(13392-28-4) |
Safety information for 1-AdaMantanethylaMine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-AdaMantanethylaMine
1-AdaMantanethylaMine manufacturer
New Products
Paroxetine Impurity G/Paroxetine Related Compound E 1-Aminocyclopentane carbonitrile (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid N,N CARBONYL DIIMIDAZOLE 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) Benzyl (3R,4S)-3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate (R)-1-Benzyl-3-pyrrolidinecarbonitrile Betahistine EP Impurity C Cyclobenzaprine N-oxide/Citalopram Related Compound E Chlorthalidone Impurity I Carbamazepine EP Impurity G Sumatriptan Succinate USP Related Compound C 2,2'-(5-methyl-1,3-phenylene)-di(2-Methylpropionitrile) 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene 5-Methyl-1,3-benzenediacetonitrile (R)-BoroLeu-(+)-Pinanediol-CF3COOH 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 4-Bromo Benzylcyanide 3-Hydroxypropionitrile 3,4 Dimethoxy Benzylcyanide valeronitrile 3-chlorobenzyl cyanide 2-Chloro BenzylcyanideRelated products of tetrahydrofuran
![Tricyclo[3.3.1.13,7]decane-1-methanamine, α-(1-methylethyl)-](https://img.chemicalbook.in/CAS/20211123/GIF/752153-58-5.gif)
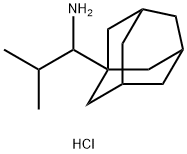
You may like
-
 2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 Sumatriptan Succinate USP Related Compound C NLT 95%View Details
2847776-12-7 -
 1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) Paroxetine Impurity G/Paroxetine Related Compound E NLT 95%View Details
1012886-75-7(HCl Salt)/69675-10-1(Freebase) -
 2856-63-5 99%View Details
2856-63-5 99%View Details
2856-63-5 -
 3,4 Diethoxy Benzylcyanide 99%View Details
3,4 Diethoxy Benzylcyanide 99%View Details
27472-21-5 -
 Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
Bromoacetaldehyde Dimethyl Acetal (stabilized with K2CO3)View Details
7252-83-7 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 (R)-1-Benzyl-3-pyrrolidinecarbonitrile 98+View Details
157528-56-8 -
 5-azidovalericacidView Details
5-azidovalericacidView Details
79583-98-5
