ε-Caprolactone
Synonym(s):2-Oxepanone;ε-Caprolactone;gamma-Caprolactone;6-Hexanolactone;6-Caprolactone monomer
- CAS NO.:502-44-3
- Empirical Formula: C6H10O2
- Molecular Weight: 114.14
- MDL number: MFCD00003267
- EINECS: 207-938-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2026-01-13 11:14:17
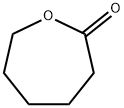
What is ε-Caprolactone?
Chemical properties
Clear colorless liquid, yellowing on ageing
The Uses of ε-Caprolactone
ε-Caprolactone is widely used as a monomer in the manufacturing of highly specialized polymers. It is mainly utilized as a precursor to caprolactam. It finds an application in the synthesis of polyglecaprone, which is used as a suture material in surgery.
The Uses of ε-Caprolactone
Intermediate in adhesives, urethane coatings, and elastomers; solvent; diluent for epoxy resins; synthetic fibers; organic synthesis.
The Uses of ε-Caprolactone
ε-caprolactone is popularly used in the preparation of poly caprolactone (PCL).
What are the applications of Application
ε-Caprolactone is a lactone building block used for the preparation of titanium alkoxides based on benzotriazole phenoxide ligands
Definition
ChEBI: A epsilon-lactone that is oxepane substituted by an oxo group at position 2.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 4079, 1958 DOI: 10.1021/ja01548a063
The Journal of Organic Chemistry, 61, p. 4560, 1996 DOI: 10.1021/jo952237x
Tetrahedron Letters, 17, p. 2455, 1976 DOI: 10.1016/0040-4039(76)90018-6
General Description
ε-caprolactone is a cyclic ester. Ring opening polymerization of ε-caprolactone initiated by, tributylin n butoxide, ethyl magnesium bromide, or samarium acetate or heteropolyacid, leads to the formation of poly caprolactone (PCL).
Flammability and Explosibility
Not classified
Properties of ε-Caprolactone
| Melting point: | -1 °C |
| Boiling point: | 96-97.5 °C10 mm Hg(lit.) |
| Density | 1.03 g/mL at 25 °C(lit.) |
| vapor density | 3.9 (vs air) |
| vapor pressure | 0.01 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 229 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform, Ethyl Acetate |
| form | Liquid |
| color | Clear colorless, yellowing on aging |
| explosive limit | 1.2-9%(V) |
| Water Solubility | >1000 MG/L (20 ºC) |
| BRN | 106919 |
| CAS DataBase Reference | 502-44-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Oxepanone(502-44-3) |
| EPA Substance Registry System | 2-Oxepanone (502-44-3) |
Safety information for ε-Caprolactone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for ε-Caprolactone
| InChIKey | PAPBSGBWRJIAAV-UHFFFAOYSA-N |
New Products
DL-beta-(3-Bromophenyl)alanine Tetrabutylammonium perchlorate N,O-Dimethylhydroxylamine hydrochloride (RS)-beta-Amino-beta-(4-bromophenyl)propionic acid 2-Amino-5-bromo-4-(trifluoromethyl)pyridine(RM for Indian lab) N,N CARBONYL DIIMIDAZOLE Levothyroxine Impurity-F Montelukast EP Impurity-D/Montelukast USP Related Compound C Atorvastatin FXA Impurity/Atorvastatin Cyclic 6-Hydroxy Impurity Sodium Salt Isosulfan blue Keto N-Oxide Impurity Ivermectin Impurity F N-Nitroso des Methyl Tramadol/N-Nitroso-N-Desmethyl-Tramadol (R)-BoroLeu-(+)-Pinanediol-CF3COOH 1,4-bis(methylsulfonyl)butane 4-(5-amino-1-methyl-1h-benzoimidazol-2-yl)-butyric acid isopropyl ester. 5-Methyl-1,3-benzenediacetonitrile 4-Fluorothiophenol 1-methyl amino-2,4-dinitro benzene CSA (DL-10-Camphorsulfonic Acid) 2-Hydroxy-4-methylnicotinic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (2S)-1-((2S,3S)-3-(2-methylbutyl)-4-oxooxetan-2-yl)pentadecan-2-yl formylleucinate 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethane-1-sulfonyl chloride S-(1-(3,5-dichlorophenyl)-2,2,2-trifluoroethyl) ethanethioateRelated products of tetrahydrofuran



You may like
-
 ε-Caprolactone monomer CAS 502-44-3View Details
ε-Caprolactone monomer CAS 502-44-3View Details
502-44-3 -
 epsilon-Caprolactone monomer >99% (GC) CAS 502-44-3View Details
epsilon-Caprolactone monomer >99% (GC) CAS 502-44-3View Details
502-44-3 -
 Epsilon-caprolactone 98% CAS 502-44-3View Details
Epsilon-caprolactone 98% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone, 97% CAS 502-44-3View Details
ε-Caprolactone, 97% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3 -
 Dimethyl adipate 95% CAS 502-44-3View Details
Dimethyl adipate 95% CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3 -
 ε-Caprolactone CAS 502-44-3View Details
ε-Caprolactone CAS 502-44-3View Details
502-44-3
