Supplier Type
- Manufacturer
- Trader
- Reagent
- contract manufacturer
- custom synthesis
Supplier Region
- Bangalore(3)
- Maharashtra(45)
- Gujarat(45)
- Hyderabad(33)
- Ahmedabad(16)
- Delhi(19)
- Mumbai(70)
- Andhra Pradesh(3)
- Haryana(4)
- Himachal Pradesh(1)
- Tamil Nadu(4)
- Pune(3)
- Uttar Pradesh(4)
- Vadodara(10)
- Kolkata(3)
- Punjab(3)
- Karnataka(7)
- Telangana(2)
- New Delhi(3)
- Rajasthan(1)
- Beijing(2)
- Nagpur(1)
- West Bengal(1)
Purity
- yes
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 96.00%
- NLT 95%
- NLT 90%
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- C6H11NO2 (Isonipecotic Acid, high purity 99%)
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.99
- 99.98%
- 99.95%
- 99.91%
- 99.90%
- 99.9%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.70%
- 99.60%
- 99.53%
- >99.5%
- 99.49%
- 99.42%
- 99.29%
- 99.00%
- 99% Min
- 99% HPLC
- 99%
- >99%
- > 99%
- 99 %
- >99 %
- 98+
- 98.60%
- 98.12%
- 98.00%
- >98%
- 98%
- 98 %
- 98 - 102
- 97-99%
- 97.75%
- 97.57%
- 97.5%
- 97.00%
- 97.0%min,97%min
- 97%
- >97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 95.48%
- 95.27%
- 95.00%
- 95%
- >95%
- 94-95%
- 94.00%
- 94% to 99.6% purity
- 94% - 97%
- 94%
- 94 ( + 0.5 )
- 94 % min
- 94 % , 74 %
- 94 - 102
- 94
- >92%
- 90% - 94%
- >90%
- 90%
- 90 % Above
- 87
- 82.9% Min
- 80-90%
- 74% 77% 90% 94%
- 40 %
- 25 MG 50 MG 100 MG 250 MG EXTRA
- 20%/95% & 1.0M
- 100% Pure
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 0MG TO GRAM
- 100mg
- 1g
- 50
- 11Between 1 gm to 1 MT (As per availability)
- 5g
- 10g
- 25g
- 50g
- 100ml
- 100g
- 500g
- 500ml
- 1L
- 500kg
- 95NLT %
- 250kgs
- 25
- 200
- 25mg
- 50mg
- 250mg
- 500mg
- 1gm
- 1and gm
- 250250 mg.
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 1kg
- 5kg
- 10kg
- 25kg
- 100kg
- 1MT
- 100
- 50kg
- 1drum
-
Levothyroxine Impurity 29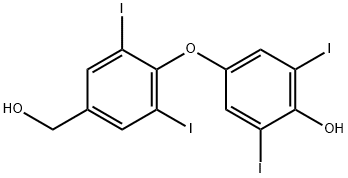
2055-94-9
-
1,1-Cyclopropanedicarboxamide,N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]phenyl]-N'-phenyl-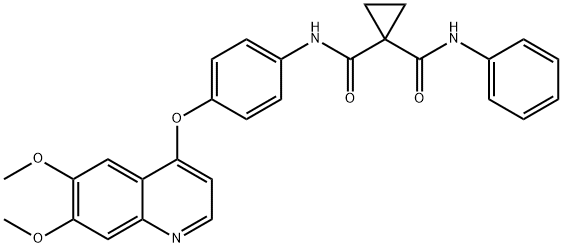
849221-94-9
-
N-[2-(DiphenylMethoxy)ethyl]-2,3,6,7-tetrahydro-N,N,1,3-tetraMethyl-2,6-dioxo-1H-purin-8-aMiniuM Inner Salt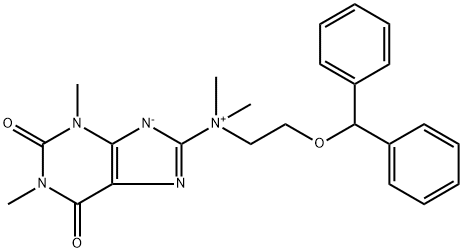
64487-94-1
-
Posaconazole Impurity 51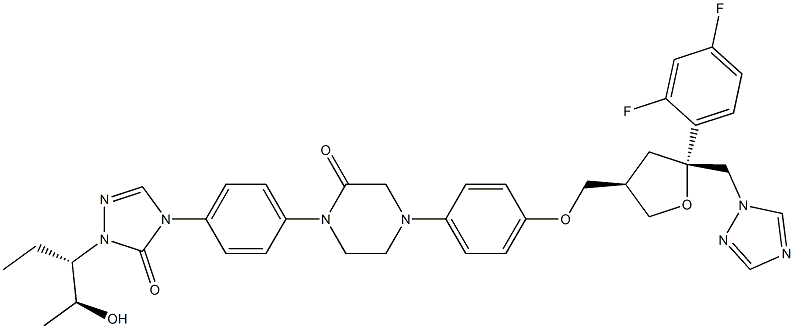
357189-94-7
-
Quetiapine Dimer Impurity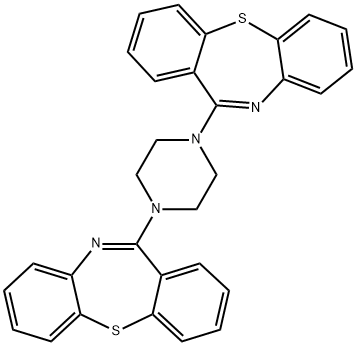
945668-94-0
-
Propanoic acid, 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methyl-, butyl ester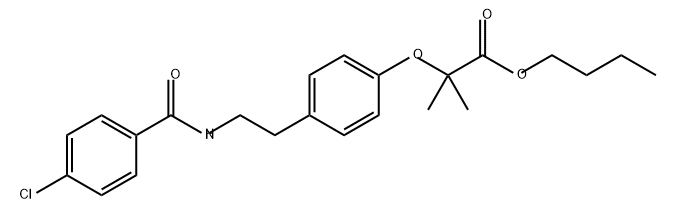
2732346-94-8
-
DL-NORPHENYLEPHRINE HYDROCHLORIDE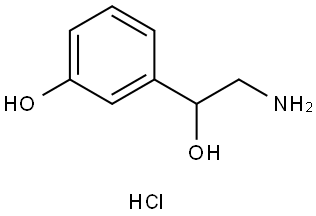
4779-94-6
-
Nor Lidocaine Hydrochloride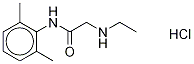
7729-94-4
-
Pyrido[2,3-d]pyrimidin-7(8H)-one, 6-acetyl-8-cyclohexyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridinyl]amino]-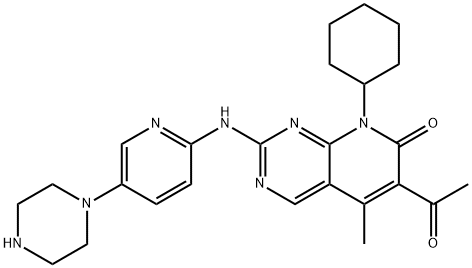
2624141-94-0
-
Glycopyrrolate IMpurity I
Levothyroxine alcohol impurity 2055-94-9
Cabozantinib Des Fluoro Impurity 849221-94-9
DIMENHYDRINATE IMPURITY 1 64487-94-1
Palbociclib Impurity C/Palbociclib N-Cyclohexyl Analog 2624141-94-0

Benzocaine 94-09-7

Triethylene glycol bis(2-ethylhexanoate) 94-28-0

Conivaptan hydrochloride 168626-94-6

Benzalacetophenone 94-41-7

Oxaliplatin 61825-94-3

Isonipecotic acid 498-94-2





