Supplier Type
- Reagent
- Manufacturer
- Trader
- contract manufacturer
- custom synthesis
Supplier Region
- Ahmedabad(16)
- Mumbai(53)
- Hyderabad(24)
- Maharashtra(37)
- Pune(7)
- Gujarat(39)
- Kerala(1)
- Tamil Nadu(5)
- Delhi(19)
- Andhra Pradesh(3)
- Karnataka(5)
- Bangalore(2)
- Telangana(4)
- Vadodara(11)
- Haryana(5)
- Punjab(2)
- New Delhi(3)
- Beijing(3)
- Kolkata(4)
- Uttar Pradesh(9)
- Rajasthan(1)
- Nagpur(1)
- Madhya Pradesh(1)
Purity
- Zn 21%
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- N20.5-21%
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Liquid 28%
- Greater than 99%
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.9%
- 99.9
- 99.87%
- 99.86%
- 99.85%
- 99.83%
- 99.80%
- 99.79%
- 99.73%
- 99.69%
- 99.5%
- 99.42%
- 99.36%
- 99.35%
- 99.29%
- 99.00%
- 99% Pure
- >99%
- 99%
- 98+
- 98.84%
- 98.60%
- 98.12%
- 98.00%
- >98%
- 98%
- 97-99%
- 97.57%
- 97.50%
- 97.00%
- >97%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96%
- 96 %
- 95.00%
- 95%
- >95%
- 94.69%
- 94.00%
- 94
- >92%
- >90%
- 90%
- 90 % Above
- 88-92%
- 87%
- 50.00%
- 50%
- 5%
- 40
- 35%
- 30% / 50%
- 30%
- 28-30 %
- 25%
- 25 MG 50 MG 100 MG 250 MG EXTRA
- 21%/25%
- 21%
- 1-35%
- 12%
- 100%
- 10%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 25
- 200
- 100
- 0Jumbo Bags or as per customer requirements
- 50
- 0KG
- 10mg
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 5g
- 10g
- 25g
- 50g
- 100g
- 100ml
- 500g
- 500ml
- 1L
- 1kg
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1MT
- 1and gm
- 50kgs
- 95NLT %
- 250kgs
- 250250 mg.
-
Sofosbuvir IMpurity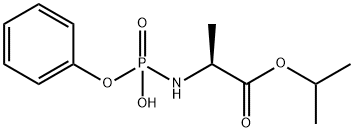
1520010-86-9
-
Sofosbuvir Impurity22
-
1-((2S,3R,4R,5R)-3-fluoro-4-hydroxy-5-(hydroxymethyl)-3-methyltetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione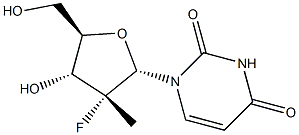
2041584-99-8
-
Sofosbuvir Impurity 113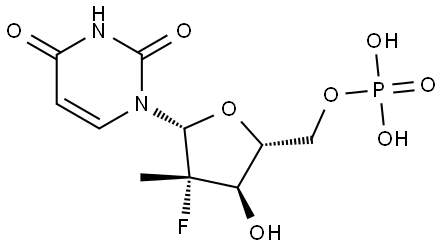
1987946-79-1
-
(S)-ethyl 2-(((S)-(perfluorophenoxy)(phenoxy)phosphoryl)amino)propanoate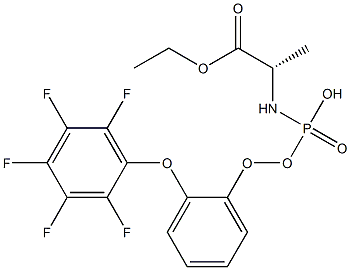
1392015-05-2
-
2'-C-Methyl-, 2',3',5'-tribenzoateuridine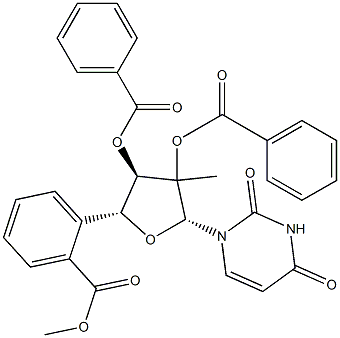
23643-36-9
-
(R)-isopropyl 2-(((R)-(perfluorophenoxy)(phenoxy)phosphoryl)amino)propanoate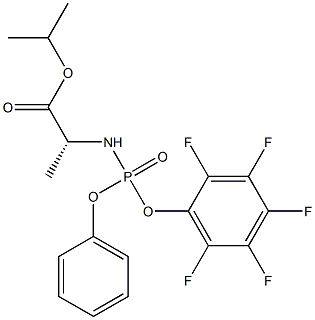
1627824-09-2
-
(S)-methyl 2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoate
1394157-34-6
-
(R)-isopropyl 2-(((S)-(perfluorophenoxy)(phenoxy)phosphoryl)aMino)propanoate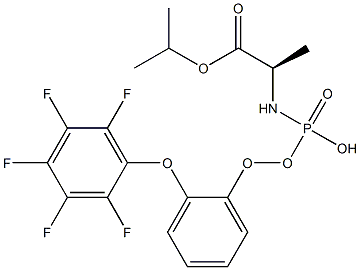
1714114-25-6
-
(S)-benzyl 2-(8-chloroimidazo[1,5-a]pyrazin-3-yl)pyrrolidine-1-carboxylate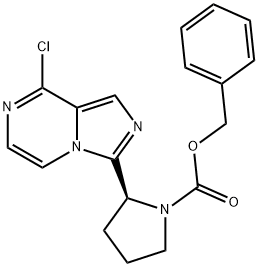
1418307-18-2

Albendazole 54965-21-8

Albendazole 54965-21-8

Betamethasone 21-phosphate sodium 151-73-5

Diallyl maleate 999-21-3

Sodium salicylate 54-21-7

6-Chloro-m-Anisidine hydrochloride 85006-21-9

Solvent yellow 21 5601-29-6

Albendazole 54965-21-8

1-(4'-Sulfophenyl)-3-methyl-5-pyrazolone 28110-21-6

Butenafine 101828-21-1

