Supplier Type
- Manufacturer
- Trader
- Reagent
Supplier Region
- Hyderabad(7)
- Maharashtra(1)
- Rajasthan(1)
- Mumbai(4)
- Gujarat(3)
- Ahmedabad(1)
- Bangalore(1)
- Pune(2)
- Punjab(1)
- Karnataka(1)
- Nagpur(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.42%
- 99.29%
- 99%
- 98.60%
- 98.12%
- 98%
- 97-99%
- 97.57%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 95.48%
- 95.00%
- >95%
- 95%
- 94.00%
- >92%
- >90%
- 90%
- 90 % Above
- 100%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 5g
- 25g
- 1kg
- 5kg
- 10kg
- 25kg
- 1and gm
- 1MT
- 250250 mg.
-
SalMeterol EP IMpurity D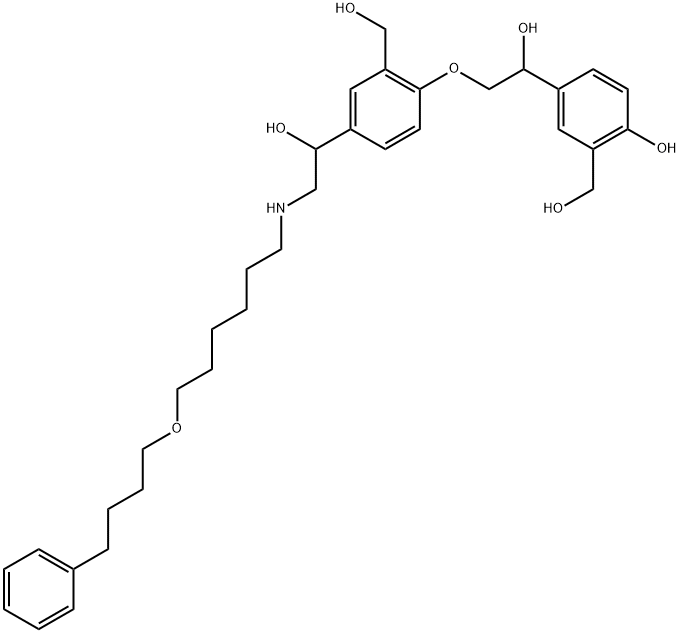
1391052-04-2
-
Salmeterol Related Compound B (10 mg) (4-{1-Hydroxy-2-[6-(4-phenylbutan-2-yloxy)hexylamino]ethyl}-2-(hydroxymethyl)phenol)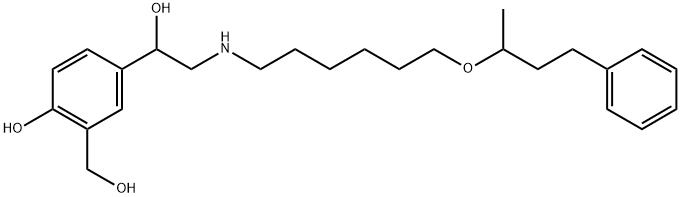
108928-81-0
-
SalMeterol EP IMpurity F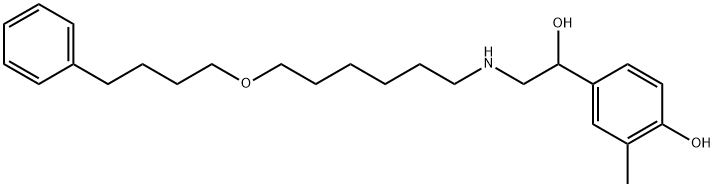
1391054-40-2
-
Salmeterol-N-Alkyl Impurity (Usp)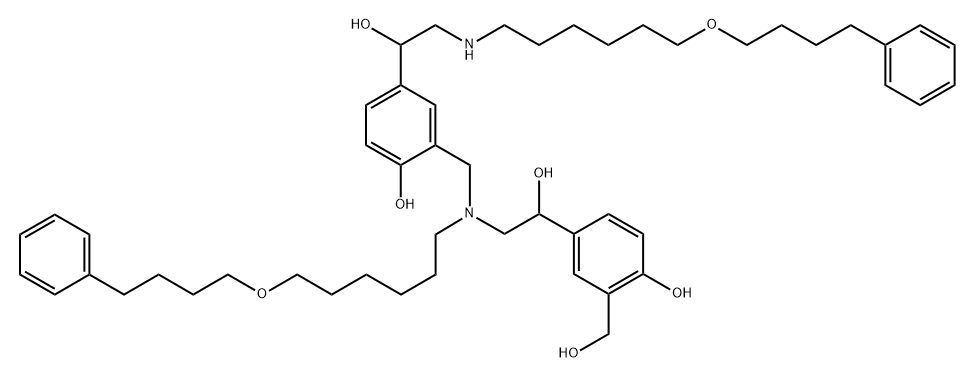
2470130-36-8
-
4-Hydroxy-α1-[[[6-(2-phenylethoxy)hexyl]aMino]Methyl]-1,3-benzenediMethanol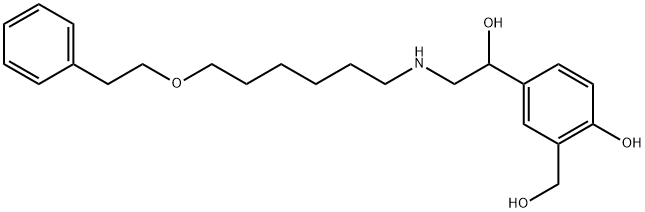
94749-02-7
-
Salmeterol EP Impurity A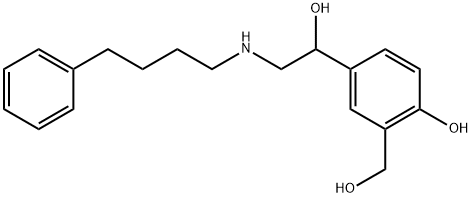
1798014-51-3
-
Salmeterol xinafoate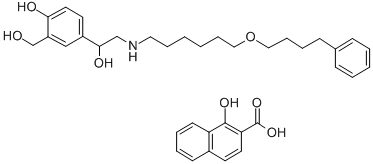
94749-08-3
-
SALMETEROL-D3 (3-HYDROXYMETHYL-D2, ALPHA-D1)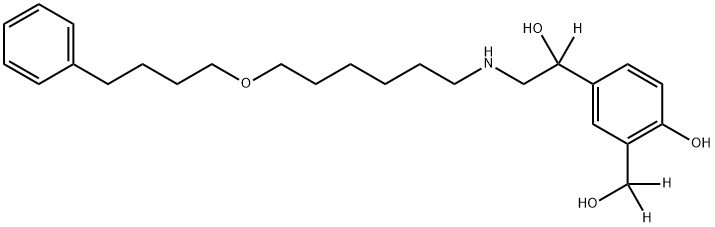
497063-94-2
-
4-Hydroxy-α1-[[[6-(3-phenylpropoxy)hexyl]aMino]Methyl]-1,3-benzenediMethanol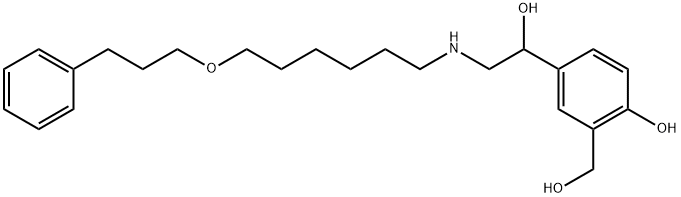
94749-11-8
-
4-[2,2-dideuterio-2-[[1,1-dideuterio-6-(4-phenylbutoxy)hexyl]amino]-1-hydroxyethyl]-2-(hydroxymethyl)phenol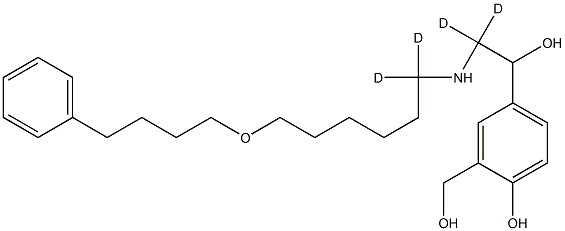
1204192-41-5
98% Granisetron EP Impurity-5, OR Impurity F, 25mg 1364914-39-5
Irinotecan Impurity C 947687-02-7
Temozolomide 2-Azahypoxanthine Impurity 4656-86-4