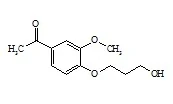
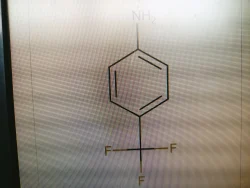
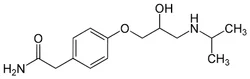
Supplier Type
- Manufacturer
- contract manufacturer
- Trader
- Reagent
Supplier Region
- Gujarat(5)
- Maharashtra(2)
- Vadodara(1)
- Ahmedabad(2)
- Mumbai(3)
- Bangalore(1)
- Pune(2)
- Hyderabad(5)
- Punjab(1)
- Karnataka(1)
- Nagpur(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.42%
- 99.29%
- 99%
- 98.60%
- 98.12%
- 98%
- 97.57%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 95.48%
- 95.00%
- >95%
- 95%
- 94.00%
- >92%
- >90%
- 90%
- 90 % Above
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 5g
- 25g
- 1kg
- 5kg
- 10kg
- 25kg
- 1and gm
- 1MT
- 250250 mg.
-
Levobupivacaine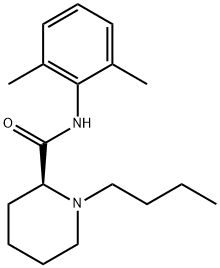
27262-47-1
-
Ropivacaine-cycle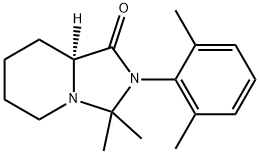
1945965-95-6
-
Ropivacaine EP Impurity D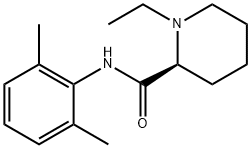
98626-59-6
-
Ropivacaine-iPr-S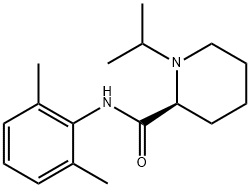
265120-58-9
-
Dexivacaine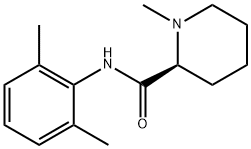
24358-84-7
-
Ropivacaine hydrochloride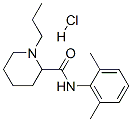
98717-15-8
-
Ropivacaine impurity G
98717-16-9
-
(S)-ropivacaine hydrochloride hydrate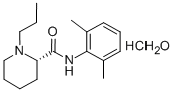
132112-35-7
-
(2S)-N-(2,6-Dimethylphenyl)-2-piperidinecarboxamide)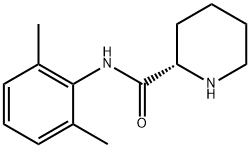
27262-40-4
-
Ropivacaine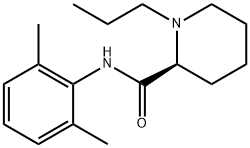
84057-95-4
ROPIVACAINE EP IMPURITY A 27262-47-1

Ropivacaine hydrochloride 98717-15-8

Ropivacaine hydrochloride 98717-15-8
Atorvastatin impurity 1371615-55-2