Supplier Type
- Manufacturer
- Reagent
- Trader
- custom synthesis
- contract manufacturer
Supplier Region
- Hyderabad(17)
- Mumbai(17)
- Gujarat(16)
- Bangalore(2)
- Maharashtra(6)
- Pune(2)
- Nagpur(1)
- Vadodara(3)
- New Delhi(1)
- Andhra Pradesh(1)
- Telangana(1)
- Ahmedabad(7)
- Karnataka(6)
- Uttar Pradesh(4)
- Delhi(7)
- Kolkata(3)
- Punjab(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- More than 98%
- more than 95%
- more than 90%
- MINIMUM 99%
- Min 98 %
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.91%
- 99.88%
- 99.86%
- 99.85%
- 99.84%
- 99.81%
- 99.80%
- 99.73%
- 99.70%
- 99.69%
- 99.61%
- 99.47%
- 99.42%
- 99.40%
- 99.39%
- 99.29%
- 99.28%
- 99.27%
- 99.21%
- 99.19%
- 99.10%
- 99.00%
- 99.0%
- 99% min
- >99%
- 99%
- > 99%
- 99 %
- 98=%
- 98+
- 98.94%
- 98.88%
- 98.65%
- 98.60%
- 98.54%
- 98.53%
- 98.50%
- 98.5
- 98.34%
- 98.18%
- 98.12%
- 98.08%
- 98.00%
- 98% Min.
- 98% Min
- >98%
- 98%
- 98 % min
- 97-99%
- 97.61%
- 97.57%
- > 97.5 %
- 97.00%
- 97% min
- 97%
- >97%
- 97 %
- 96.80%
- > 96.5 %
- 96.5 - 103.5 %
- 96.40%
- 96.39%
- 96.14%
- 96%
- 95-98%
- 95.48%
- 95.16%
- 95.00%
- 95%
- >95%
- 94.34%
- 94.00%
- 92-95%
- >92%
- 91.87%
- 90%-95%
- 90%
- >90%
- 90 % Above
- 89%
- 85 percent Min
- 84.49%
- 100%
- 0.04%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1gm
- 1g
- 5g
- 10g
- 25g
- 50g
- 100g
- 500g
- 1kg
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1and gm
- 1MT
- 250250 mg.
- 11Between 1 gm to 1 MT (As per availability)
-
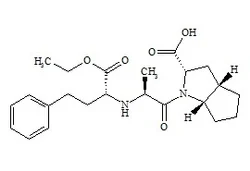
[2S-[1[R*(R*)],2α,3aβ,6aβ]]-1-[2-[[3-Cyclohexyl-1-(ethoxycarbonyl)propyl]aMino]-1-oxoprop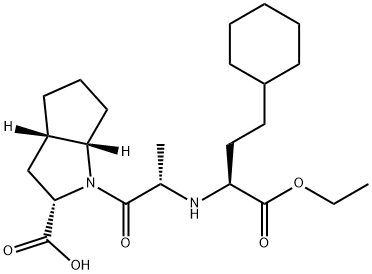
99742-35-5
-
RaMipril EP IMpurity H
-
(α1S,α4S,2S,5S)-2,5-DiMethyl-3,6-dioxo-α1,α4-bis(2-phenylethyl)-1,4-piperazinediacetic Acid 1,4-Diethyl Ester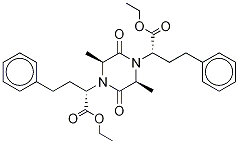
151387-05-2
-
Ramiprilat Diketopiperazine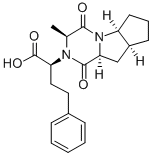
108736-10-3
-
Ramiprilat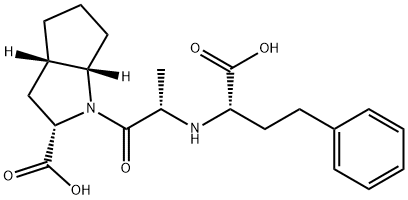
87269-97-4
-
Ramipril Related Compound A (30 mg) ((2S,3aS,6aS)-1-[(S)2-[[(S)-1-(methoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-octahydrocyclopenta[b]pyrrole-2-carboxylic acid)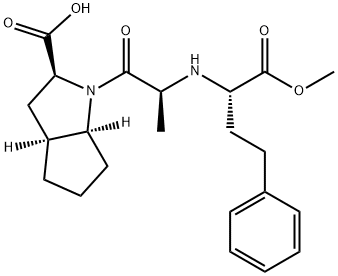
108313-11-7
-
2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER
87269-88-3
-
N-[(S)-(+)-1-(Ethoxycarbonyl)-3-phenylpropyl]-L-alanine
82717-96-2
-
1H-Indole-6-carboxylic acid, 2,3-dihydro-3-[[(4-hydroxyphenyl)amino]phenylmethylene]-2-oxo-, methyl ester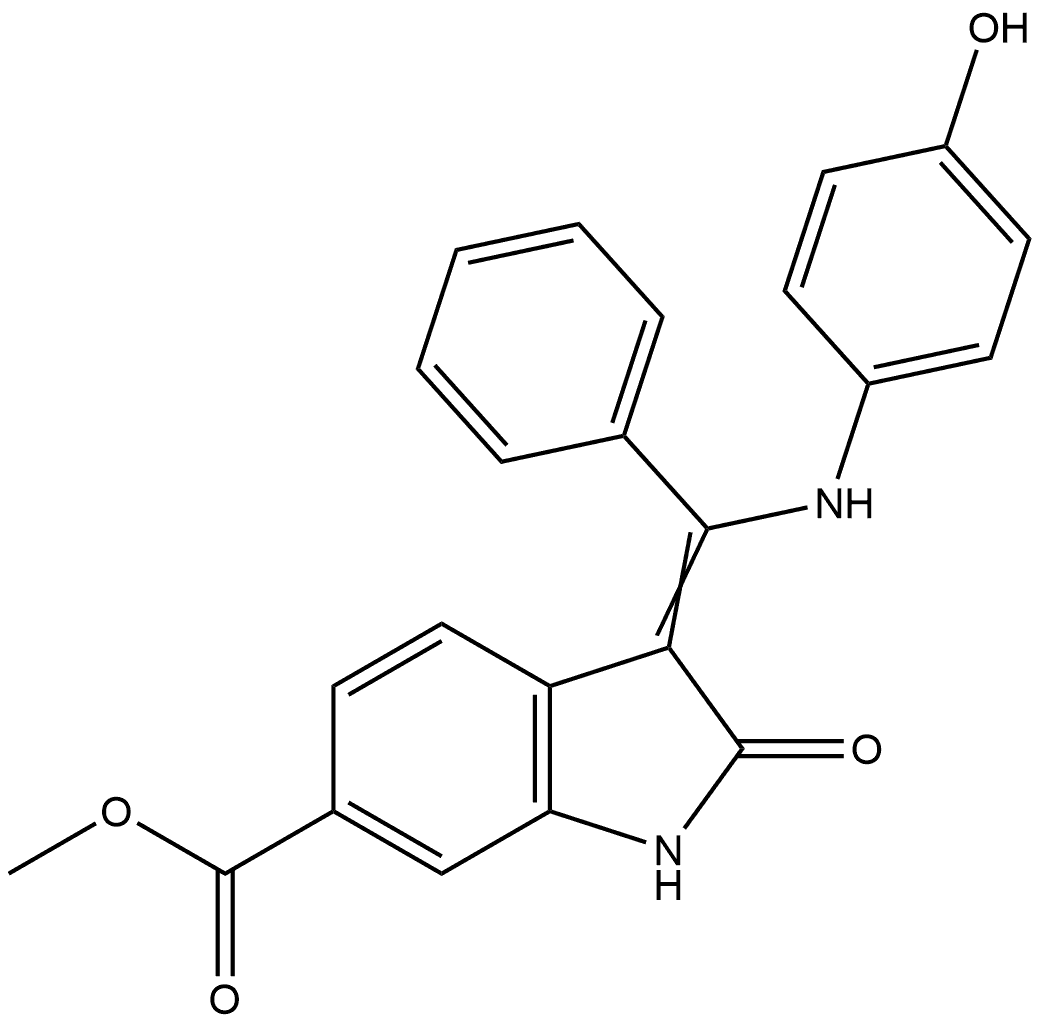
2519501-85-8
>92% Ramipril EP Impurity C, 100mg, Industrial Grade 99742-35-5
RAMIPRIL EP IMPURITY O 151387-05-2
Etoposide impurity C 100007-53-2
Irinotecan Impurity C 947687-02-7
Fosaprepitant Impurity C 170729-80-3
99% Lamivudine EP Impurity C 69-72-7