Supplier Type
- Manufacturer
- Reagent
- Trader
- contract manufacturer
- custom synthesis
Supplier Region
- Maharashtra(38)
- Mumbai(58)
- Bangalore(4)
- Hyderabad(33)
- Gujarat(46)
- Karnataka(3)
- Tamil Nadu(3)
- Uttar Pradesh(5)
- Ahmedabad(15)
- Andhra Pradesh(1)
- Vadodara(9)
- Himachal Pradesh(1)
- Kolkata(1)
- Delhi(8)
- Telangana(2)
- Ujjain(1)
- Pune(4)
- New Delhi(5)
- Kerela(1)
- Punjab(2)
- Beijing(1)
- Rajasthan(1)
- Nagpur(1)
- West Bengal(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- NLT 95%
- NLT 90%
- more than 95%
- more than 90%
- Min. 40.0 %
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.99
- 99.98%
- 99.95%
- 99.91%
- 99.9%
- 99.9 %
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.64%
- 99.5% Min
- 99.5%
- 99.42%
- 99.29%
- 99.20%
- 99.00%
- 99% Pure
- 99% HPLC
- 99%
- >99%
- 99 %
- 99
- 98+
- 98.60%
- 98.12%
- 98.00%
- 98%+
- 98% to 101%
- >98%
- 98%
- 98
- 97-99%
- 97.93%
- 97.57%
- 97.00%
- 97% min
- >97%
- 97%
- 97 %
- 96.80%
- 96.39%
- 96.25%
- 96.14%
- 96%
- 95-99%
- 95.48%
- 95.03%
- 95.00%
- 95% & 2.0M
- >95%
- 95%
- 94.00%
- 93%
- >92%
- 90%
- >90%
- 90 % Above
- 72.96%
- 50%
- 46%
- 25 MG 50 MG 100 MG 250 MG EXTRA
- 100% Pure
- 100%
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 100mg
- 25
- 500mg
- 200
- 25mg
- 50mg
- 100
- 250mg
- 1gm
- 1g
- 5g
- 10g
- 100ml
- 5kg
- 2.5501e+00925 MG 50 MG 100 MG 250 MG EXTRA
- 25g
- 50g
- 100g
- 500g
- 500ml
- 1kg
- 1L
- 10kg
- 25kg
- 50Kg
- 100kg
- 500kg
- 1and gm
- 1MT
- 95NLT %
- 250kgs
- 250250 mg.
- 50
-
Lenacapavir Impurity 46
-
Lenacapavir Impurity 49
3053923-46-6
-
Lenacapavir Impurity 53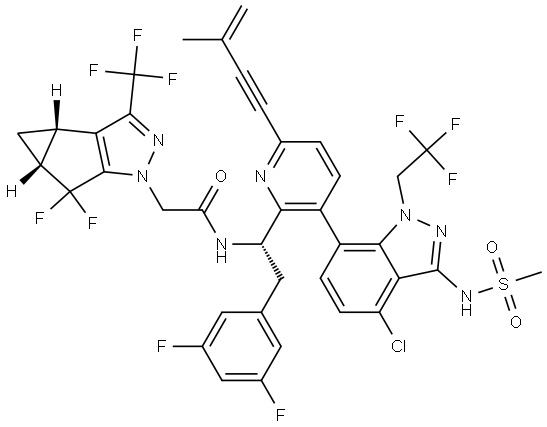
3025145-45-0
-
Lenacapavir Impurity 50
-
Lenacapavir Impurity 54
3025145-44-9
-
N-((S)-1-(3-(3-amino-4-chloro-1-(2,2,2-trifluoroethyl)-1H-indazol-7-yl)-6-(3-methyl-3-(methylsulfonyl)but-1-yn-1-yl)pyridin-2-yl)-2-(3,5-difluorophenyl)ethyl)-2-((3bS,4aR)-5,5-difluoro-3-(trifluoromethyl)-3b,4,4a,5-tetrahydro-1H-cyclopropa[3,4]cyclopenta[1,2-c]pyrazol-1-yl)acetamide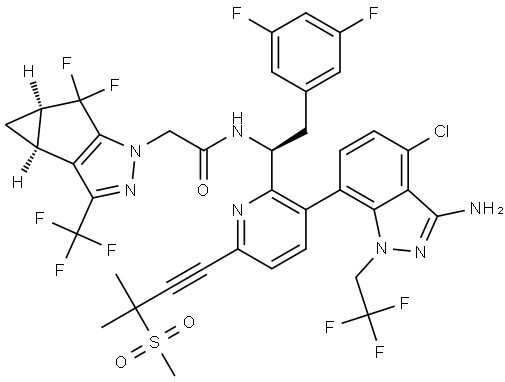
2375020-20-3
-
Lenacapavir Impurity 51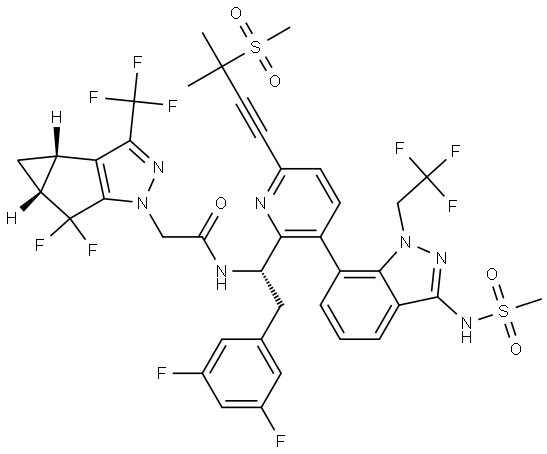
3025145-47-2
-
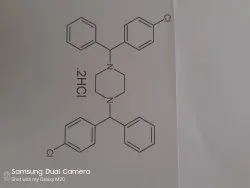
1,4-Bis[(4-chlorophenyl)-phenylMethyl]piperazine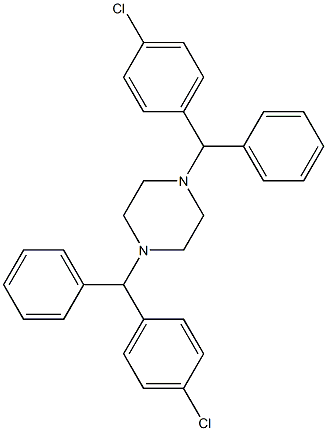
-
Cetirizine EP Impurity C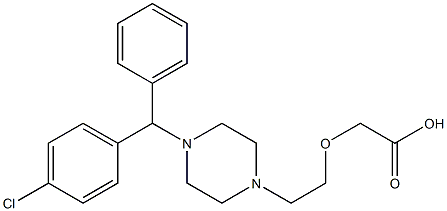
-
IpratropiuM BroMide IMpurity F (Mixture of DiastereoMers)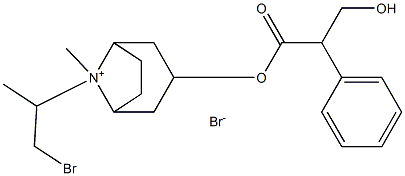
17812-46-3
99.29% Powder Cetirizine EP Impurity D, 246870-46-2 246870-46-2
Ipratropium bromide impurity 17812-46-3
Glimepiride Impurity -A 684286-46-2
OXCARBAZEPINE EP IMPURITY A 298-46-4

Zinc Citrate 546-46-3

3-Amino-1H-pyrazole-4-carbonitrile 16617-46-2

Peg-7 glyceryl cocoate 68201-46-7

(4-Methylphenoxy)acetaldehyde 67845-46-9

Zinc Citrate 546-46-3