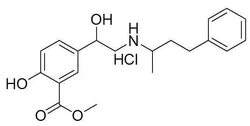
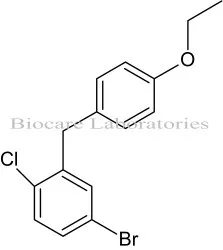
- bulk
Supplier Type
- Manufacturer
- Trader
- contract manufacturer
- Reagent
- custom synthesis
Supplier Region
- Bangalore(3)
- Tamil Nadu(1)
- Mumbai(18)
- Maharashtra(12)
- Andhra Pradesh(2)
- Hyderabad(15)
- Delhi(2)
- Ahmedabad(3)
- Gujarat(11)
- Telangana(1)
- Pune(3)
- Karnataka(4)
- Uttar Pradesh(1)
- Vadodara(1)
- Nagpur(1)
- Punjab(1)
Purity
- Salicylic Acid
- Rasagiline Mesylate Impurity C
- more than 95%
- more than 90%
- Mesalazine EP Impurity H
- Mathadone (M225865) impurity. Used in synthesis of methadone, antispasmodics and other pharmaceutica
- Greater than 99%
- Empagliflozin intermediate, Dapagliflozin Impurity
- Dipropargyl r- amino indane
- Dapagliflozin Intermediate, Dapagliflozin Impurity
- API Impurity / In-house working standard
- Acetylsalicylic Acid EP Impurity C
- 99.99%
- 99.98%
- 99.95%
- 99.93%
- 99.91%
- 99.90%
- 99.87%
- 99.86%
- 99.85%
- 99.80%
- 99.73%
- 99.63%
- 99.42%
- 99.29%
- 99% min
- 99%
- 99 %
- 98.60%
- 98.57%
- 98.12%
- 98.00%
- 98%+
- 98%
- 98 %
- 97-99%
- 97.57%
- 97.00%
- 97%
- >97%
- 97 %
- 96.80%
- 96.39%
- 96.14%
- 96%
- 95.48%
- 95.00%
- 95%
- >95%
- 94.00%
- >92%
- 90%
- >90%
- 90 % Above
- (R)-N,N-di(prop-2-yn-1-yl)-2,3- dihydro-1
Package
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1g
- 1gm
- 5g
- 10g
- 25g
- 50g
- 100g
- 500g
- 1kg
- 5kg
- 10kg
- 25kg
- 50kg
- 100kg
- 500kg
- 1MT
- 1and gm
- 250250 mg.
-
Atorvastatin Epoxy Tetrahydrofuran IMpurity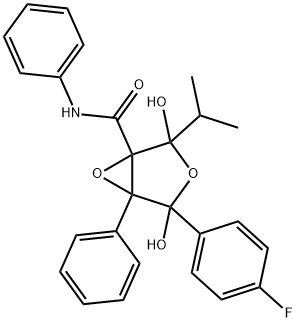
873950-19-7
-
Atorvastatin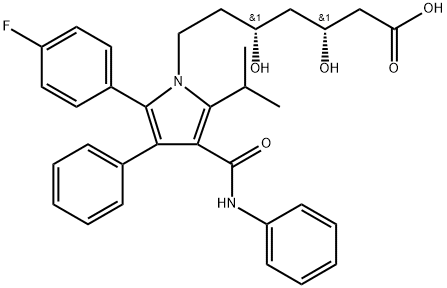
134523-00-5
-
1,5-Dihydroxy naphthalene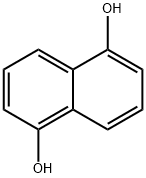
83-56-7
-
Atorvastatin calcium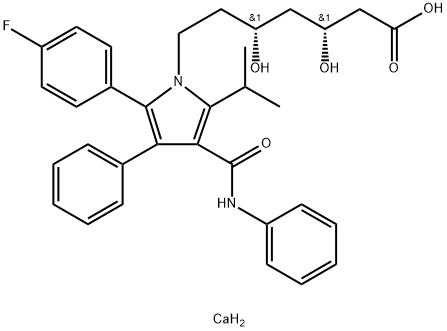
134523-03-8
-
DL-Tartaric acid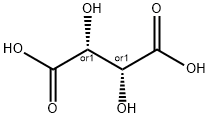
133-37-9
-
2',5'-Dihydroxyacetophenone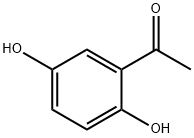
490-78-8
-
Minocycline hydrochloride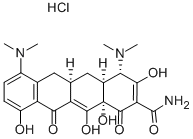
13614-98-7
-
Etoposide Impurity 9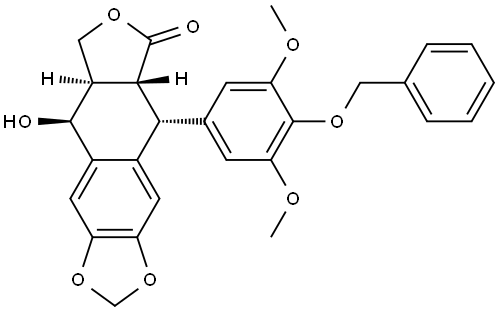
403642-80-8
-
Darunavir Amine dimer impurity
-
methyl[(naphthalen-1-yl)methyl]nitrosoamine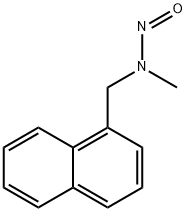
296760-88-8

Atorvastatin calcium 134523-03-8

3,4-Dihydroxy-5-nitro-benzaldehyde 116313-85-0
Atorvastatin Calcium Api, Grade Standard: IP 134523-03-8

Atorvastatin calcium 134523-03-8

Atorvastatin 134523-00-5

Dihydroxy Acetone 96-26-4