CHEMICAL AND PHYSICAL PROPERTIES
| Stability/Shelf Life | Stable if stored as directed; avoid strong oxidizing agents |
|---|---|
| Decomposition | Dangerous products of decomposition: thermal ecomposition may produce toxic gases such as carbon monoxide, carbon dioxide, and nitrogen oxides. |
| Dissociation Constants | 2.77 and 7.8 |
| Other Experimental Properties | Most amides hydrolyze to acids extremely slowly at 25 °C and pH 7 with half-lives measured in centuries. |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
COMPUTED DESCRIPTORS
| Molecular Weight | 532.6 g/mol |
|---|---|
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 532.21984399 g/mol |
| Monoisotopic Mass | 532.21984399 g/mol |
| Topological Polar Surface Area | 65.8 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 910 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
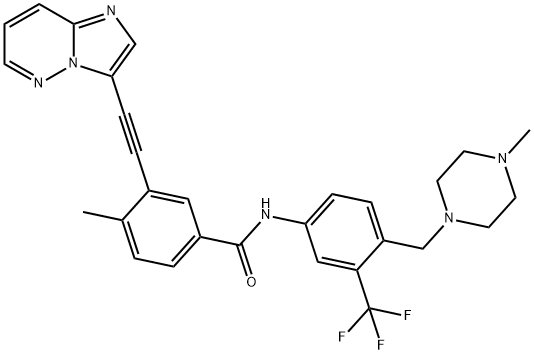
Ponatinib is a benzamide obtained by the formal condensation of the carboxy group of 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methylbenzoic acid with the anilino group of 4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)aniline. It is a multi-target tyrosine kinase inhibitor that targets ABL, SRC, FGFR, and others and was designed to overcome the resistance of BCR-ABL mutation to imatinib, in particular the gatekeeper mutation ABL(T315I). It has a role as an antineoplastic agent and a tyrosine kinase inhibitor. It is a N-methylpiperazine, a member of benzamides, an acetylenic compound, an imidazopyridazine and a member of (trifluoromethyl)benzenes. It is a conjugate base of a ponatinib(1+).