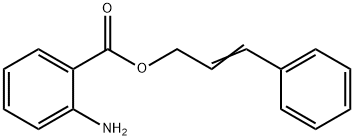
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Cinnamyl anthranilate appears as brownish or reddish-yellow powder or light yellow solid with a fruity taste. (NTP, 1992) |
|---|---|
| Color/Form | BROWNISH POWDER |
| Odor | BALSAMIC, FRUITY ODOR SOMEWHAT REMINISCENT OF GRAPE |
| Boiling Point | 630 °F at 760 mmHg (NTP, 1992) |
| Melting Point | 142 to 143 °F (NTP, 1992) |
| Solubility | less than 1 mg/mL at 63 °F (NTP, 1992) |
| Density | 1.18 at 59.9 °F (NTP, 1992) - Denser than water; will sink |
| Stability/Shelf Life | CHANGE ON EXPOSURE TO LIGHT, DUE TO PHOTOOXIDATION /AROMATIC AMINES & RELATED NITRO COMPD/ |
| Chemical Classes | Other Classes -> Esters, Other |
COMPUTED DESCRIPTORS
| Molecular Weight | 253.29 g/mol |
|---|---|
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 253.110278721 g/mol |
| Monoisotopic Mass | 253.110278721 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Cinnamyl anthranilate appears as brownish or reddish-yellow powder or light yellow solid with a fruity taste. (NTP, 1992)