83-56-7
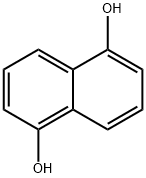
Product Name:
1,5-Dihydroxy naphthalene
Formula:
C10H8O2
Synonyms:
1,5-Naphthalenediol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Orange powder; [ICSC] |
|---|---|
| Flash Point | 252 °C |
| Solubility | Solubility in water, g/100ml at 20 °C: 0.06 |
| Vapor Pressure | 0.00000715 [mmHg] |
| LogP | 1.82 |
| Decomposition | 250 °C |
| Collision Cross Section | 134.35 Ų [M-H]- [CCS Type: DT, Method: stepped-field] |
| Chemical Classes | Other Classes -> Naphthols |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 160.17 g/mol |
|---|---|
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 160.052429494 g/mol |
| Monoisotopic Mass | 160.052429494 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 140 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
1,5-Dihydroxynaphthalene is a natural product found in Magnolia liliiflora and Sanicula lamelligera with data available.
