CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Hydrated crystals from water |
| Odor | Odorless |
| Taste | Bitter |
| Boiling Point | 719.69 |
| Melting Point | 133-135 |
| Solubility | Soluble in water at 2mg/ml |
| LogP | 2.6 |
| LogS | -3.2 |
| Optical Rotation | Hydrated crystals from acetone + water, mp 106-110 °C. Specific optical rotation = -42.5 °C at 25 °C/D /Erythromycin ethyl succinate/ |
| Decomposition | When heated to decomp it emits toxic fumes of /nitric oxides./ |
| pH | pH (saturated solution): 8 to 10.5; pH <4 is destructive |
| Ionization Efficiency | Positive |
| Refractive Index | INDEX OF REFRACTION: 1.490 (ALPHA), 1.515 (BETA), 1.567 (GAMMA) /Erythromycin ethyl succinate/ |
| Dissociation Constants | 8.8 |
| Collision Cross Section | 258.1 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | Slightly hygroscopic |
COMPUTED DESCRIPTORS
| Molecular Weight | 733.9 g/mol |
|---|---|
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 7 |
| Exact Mass | 733.46124119 g/mol |
| Monoisotopic Mass | 733.46124119 g/mol |
| Topological Polar Surface Area | 194 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1180 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
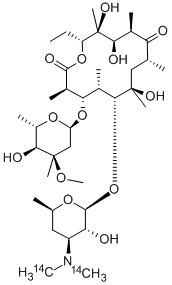
Erythromycin A is an erythromycin that consists of erythronolide A having 2,6-dideoxy-3-C-methyl-3-O-methyl-alpha-L-ribo-hexopyranosyl and 3,4,6-trideoxy-3-(dimethylamino)-beta-D-xylo-hexopyranosyl residues attahced at positions 4 and 6 respectively. It is an erythromycin and a cyclic ketone. It is functionally related to an erythronolide A. It is a conjugate base of an erythromycin A(1+).