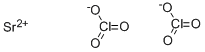CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Strontium chlorate appears as a moist solid or semi-solid slurry of white crystals. May explode under exposure to heat or fire. Used in pyrotechnics. |
|---|---|
| Color/Form | COLORLESS OR WHITE CRYSTALS |
| Melting Point | 120 °C (Decomp) |
| Solubility | 174.9 G/100 ML WATER AT 18 °C |
| Density | 3.15 g/cu-cm |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride/. |
| Refractive Index | INDEX OF REFRACTION: 1.516, 1.605, & 1.626 |
| Other Experimental Properties | DECOMPOSES AT 120 °C WITH EVOLUTION OF OXYGEN |
| Chemical Classes | Other Classes -> Inorganic Oxidizing Agents |
COMPUTED DESCRIPTORS
| Molecular Weight | 254.52 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 253.8128054 g/mol |
| Monoisotopic Mass | 253.8128054 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 36.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Strontium chlorate appears as a moist solid or semi-solid slurry of white crystals. May explode under exposure to heat or fire. Used in pyrotechnics.