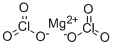CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Magnesium chlorate appears as white deliquescent crystals or powder. Soluble in water and denser than water. Poses a dangerous fire risk when in contact with organic materials or heat. May be irritating to skin, eyes and mucous membranes. Used to make other chemicals. |
|---|
COMPUTED DESCRIPTORS
| Molecular Weight | 191.20 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 189.8922348 g/mol |
| Monoisotopic Mass | 189.8922348 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 36.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 3 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Magnesium chlorate appears as white deliquescent crystals or powder. Soluble in water and denser than water. Poses a dangerous fire risk when in contact with organic materials or heat. May be irritating to skin, eyes and mucous membranes. Used to make other chemicals.