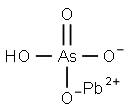CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Lead arsenate is a white powder. It is insoluble in water. It is toxic by skin absorption, inhalation and by ingestion. |
|---|---|
| Color/Form | White monoclinic crystals |
| Odor | Odorless |
| Melting Point | 280 °C (decomposes) |
| Solubility | Insoluble in water |
| Density | 5.79 at 59 °F (USCG, 1999) - Denser than water; will sink |
| Stability/Shelf Life | Stable to light, air & water & is not decomp by carbon dioxide; decomposed by alkalies; incl calcium hydroxide. |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomposition it emits very toxic fumes of /arsenic and lead/. /SRP: including arsine/ |
| Refractive Index | Index of refraction: 1.90 (alpha), 1.97 (gamma) |
| Other Experimental Properties | At about 280 °C loses water and is converted into pyroarsenate |
COMPUTED DESCRIPTORS
| Molecular Weight | 347 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 347.88573 g/mol |
| Monoisotopic Mass | 347.88573 g/mol |
| Topological Polar Surface Area | 83.4 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 46.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Lead arsenate is a white powder. It is insoluble in water. It is toxic by skin absorption, inhalation and by ingestion.